Functional analysis of AAA-ATPase bromodomain proteins
The ATPase family, AAA domain-containing protein 2 (ATAD2, or ANCCA) is an AAA nuclear co-regulator protein highly overexpressed in many cancers, including breast and prostate cancer. ATAD2 is a co-activator of the androgen and estrogen receptors, and has been involved with the proliferation of cancer cells. Additionally, higher levels of ATAD2 activity have been correlated to an increased risk of cancer metastasis and recurrence. ATAD2B is a poorly studied paralog of the ATAD2 gene, and although ATAD2 and ATAD2B are highly conserved, there is very little known about the function of ATAD2B, or its role in oncogenesis. Both the ATAD2 and ATAD2B proteins contain two conserved domains: 2 distinct AAA-ATPase domains and a bromodomain. We have discovered that the ATAD2/B bromodomains are di-acetyllysine readers, however, little is known about how these epigenetic signaling proteins are targeted to histones. The overall objective of this project is study the ATAD2/B bromodomains and their molecular functions. Our central hypothesis is the ATAD2/B bromodomains sense epigenetic signals from multiple modifications on the histone tails to regulate gene expression and cellular proliferation. The rationale for our research is that further understanding of how the ATAD2/B bromodomains recognize histone post-translational modifications (PTMs) will reveal their position in normal biological and cellular processes; and shed light on how to disrupt their role in oncogenesis.
Related papers:
https://pubs.acs.org/doi/10.1021/acs.jmedchem.0c01178

The role of bromodomain proteins and endocrine sensitivity in ER+ breast cancers
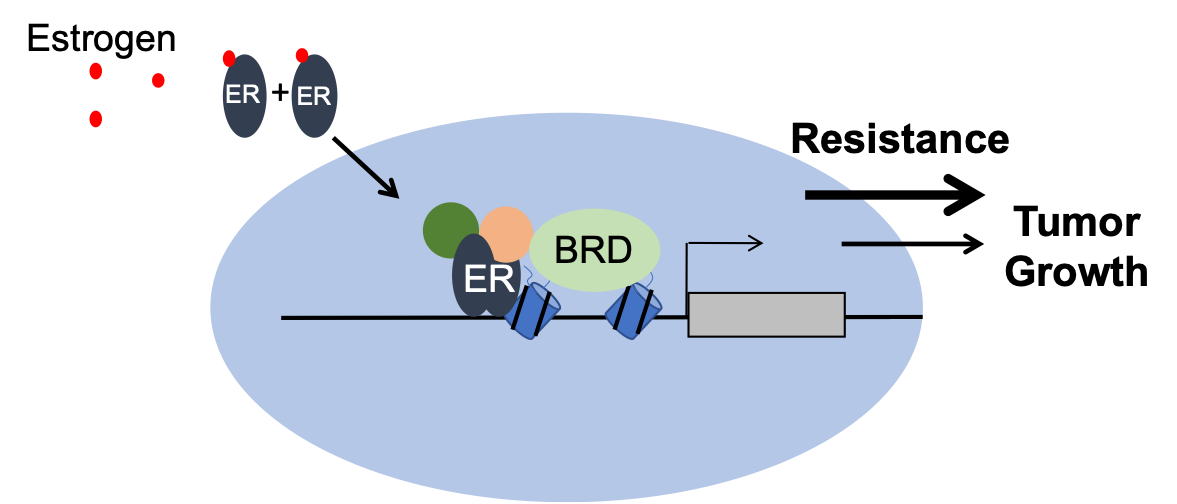
ER+ breast tumors often fail to respond to endocrine therapies. This phenomenon is driven by subpopulations of breast cancer cells that respond differently to targeted therapies than most of the bulk tumor cells. These surviving cells exhibit transcriptional plasticity in a manner that depends on epigenetic control. Combinatorial patterns of histone acetyllysine patterns govern anti-estrogen responses via enhancer regulatory maps that direct estrogen receptor (ER) actions. Bromodomains recognize patterns of acetyllysine and work together with ER and coregulators to effect transcriptional change. We hypothesize that bromodomain have a causal relationship between transcriptional plasticity and endocrine therapy resistance. Though an integrated approach using cell and patient samples, we are exploring the role of bromodomain-containing proteins and their role endocrine responsiveness in endocrine sensitivity.
Related papers:
https://www.nature.com/articles/s41467-019-09320-9
Investigating the role of Ikaros as a tumor suppressor in acute lymphoblastic leukemia
Precursor B cell acute lymphoblastic leukemia (pre-B ALL) is the most common childhood cancer. Significant advances in the treatment of leukemia in recent years have greatly improved the overall survival of ALL. However, ALL remains a leading cause of childhood cancer-related morbidity and mortality, with high-risk subgroups contributing disproportionately to the overall disease mortality. Recent high-resolution sequencing analysis of leukemia has provided valuable insight into the molecular pathways that are altered in ALL, including collaborating mutations in oncogenes and tumor suppressor genes. In particular, recurrent mutations in the IKZF1 gene encoding the tumor suppressor Ikaros has been identified in pre-B ALL, with near obligate loss-of-function lesions in the BCR-ABL1+ (Ph+) subgroup of pre-B ALL, suggesting that inactivation of Ikaros is linked to the pathogenesis of Ph+ pre-B ALL. While this association is well documented, the molecular mechanisms underlying this strong leukemic collaboration are not well known. Furthermore, IKZF1 mutations are associated with poor prognosis6-9, but the mechanisms of Ikaros tumor suppression in pre-B ALL are poorly understood. Importantly, there are currently no available treatment options that can target IKZF1-mutated ALL. In this project we will investigate the biological consequences of Ikaros deficiencies in pre-B ALL in order to provide a foundation for rational design of targeted therapies to treat patients diagnosed with IKZF1-mutated leukemia.
Related papers:
https://rupress.org/jem/article/214/3/793/42181/Genetic-analysis-of-Ikaros-target-genes-and-tumor
https://rupress.org/jem/article/214/3/773/42169/Conserved-IKAROS-regulated-genes-associated-with-B