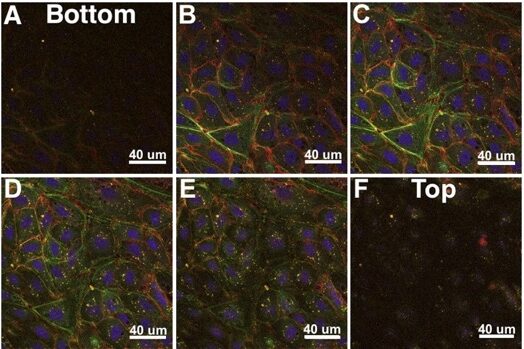
Impact of Nanoparticles on Endothelial Cell Function
Advancement in the field of nanotechnology has increased the use of nanoparticles (NPs) in many applications. NPs have many advantages, but their potential is in part hampered by the complexity of the biological response to particles. The endothelium is a primary barrier to NP transport into tissues for particles administered intravenously. In vivo, endothelial cells are also subjected to the force of the flowing blood, called shear stress (SS); therefore, it is crucial to study the effects of SS on NP-endothelial interactions. Our work shows that 20 nm NPs uniquely increase the permeability of the endothelial barrier without affecting the cell viability by altering cellular Ca2+ homeostasis. We seek to further understand the mechanism of calcium changes due to particle-endothelial interactions, with implications for human health in the presence of NPs as well as applications in drug delivery.
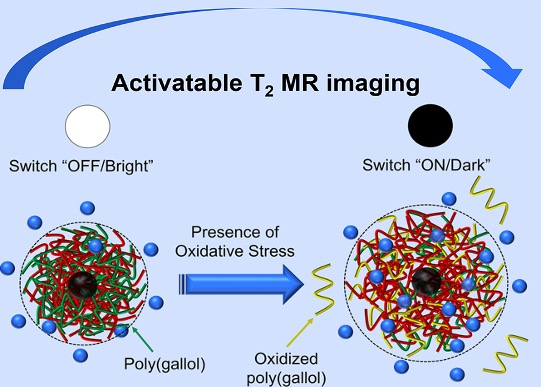
Imaging Inflammation
Inflammatory diseases include a wide range of disorders and conditions such as atherosclerosis, cancers, and glomerulonephritis. Inflammatory disorders occur when the inflammatory response becomes uncontrolled, resulting in destruction of healthy tissues. Signaling molecules such as reactive oxygen species (ROS) play a central role in chronic inflammation. Excessive ROS production at inflammatory sites triggers endothelial dysfunction and tissue injury. Proper detection and diagnosis of inflammatory conditions remains elusive despite huge clinical implications. We developed activatable magnetic resonance (MR) contrast agents sensitive to the presence of ROS to improve diagnosis of inflammatory diseases. We synthesized interpolymer complexed superparamagnetic iron oxide nanoparticles (IPC-SPIOs) targeted to macrophages that are capable of activating T2 MR signal selectively under oxidative conditions, with implications for better disease diagnosis and patient outcomes.
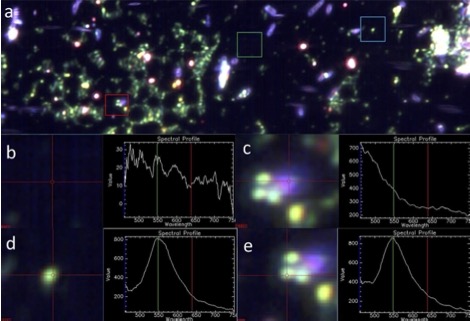
Bacterial photoablation
This project targets Pseudomonas aeruginosa, a gram negative bacteria commonly associated with bacterial keratitis, with gold nanoparticles towards the end goal of killing the bacteria through photoablation. Photoablation is a non-thermal mechanism that is activated when light hits the nanoparticles and they release toxic species. We are currently working towards a therapy for bacterial infections in the eye called bacterial keratitis that are difficult to treat and can cause blindness.
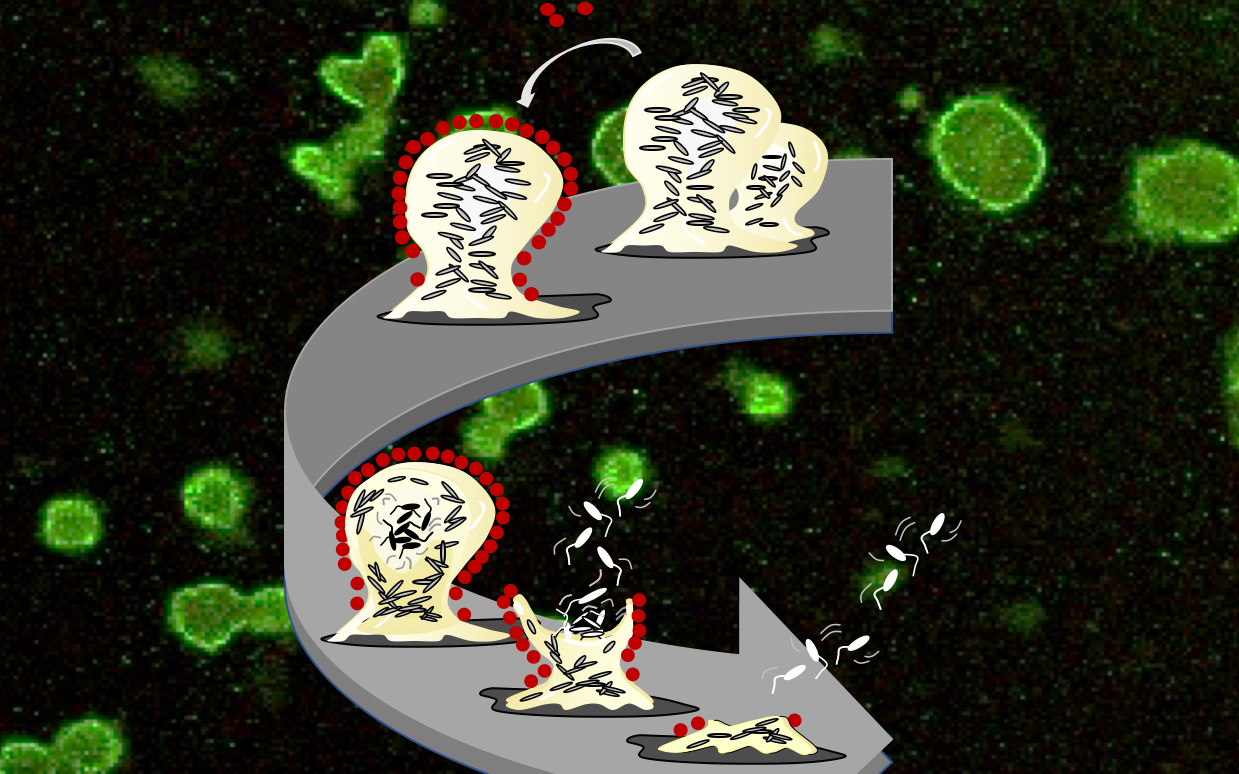
Combating Bacterial Biofilms
Despite remarkable advancements in the treatment of wounds, mortality rates remain high, with wound infections being responsible for over 200,000 deaths annually in the United States alone, which is due in large part to the challenges of treating biofilm infections. Biofilms are surface- associated, matrix-enclosed bacterial communities that are refractory to conventional antibiotic treatment and host immune responses. Along with collaborators, we have shown that depletion of pyruvate prevents biofilms from forming, induces dispersion of existing biofilms, and increases the susceptibility of biofilm cells up to 1000x to traditional antimicrobials and phagocytic cells. We are continuing to develop a wound dressing based on pyruvate removal for improved patient outcomes.