
Climate Change in Forests
Download chapter PDF and figures
Authors
Jessica Wikle
Rubenstein School of Environment and Natural Resources, Gund Institute for Environment, University of Vermont
Hanusia Higgins
Rubenstein School of Environment and Natural Resources, University of Vermont
Jamey Fidel
General Counsel, Forest and Wildlife Program Director, Vermont Natural Resources Council.
Wrote Land Use Change section
Deniz Dutton
Rubenstein School of Environment and Natural Resources, Gund Institute for Environment, University of Vermont
Elise Schadler
Department of Forests, Parks, & Recreation, Vermont Agency of Natural Resources
Wrote Urban Forests and Climate section
Alexandra Kosiba
Department of Forests, Parks, & Recreation, Vermont Agency of Natural Resources
Wrote Urban Forests and Climate section
Joanne Garton
Department of Forests, Parks, & Recreation, Vermont Agency of Natural Resources
Wrote Urban Forests and Climate section
Peter Clark
Rubenstein School of Environment and Natural Resources, University of Vermont
Wrote Boxes 2.4 and 2.7
Dani Cook
Forest Ecosystem Monitoring Cooperative, University of Vermont
Wrote Infrastructure Costs section
Citation
Higgins, H., Wikle, J., Clark, P., Cook, D., Dutton, D., Garton, J., Kosiba, A., Schadler, E., 2021. Climate Change in Forests. In Galford, G.L., Faulkner, J. et al. (Eds), The Vermont Climate Assessment 2021. Burlington, Vermont: Gund Institute for Environment at the University of Vermont. DOI: 10.18125/kowgvg.
Table of Contents
- 2.1 Key Messages
- 2.2 Forest Structure and Composition
- 2.3 Forest Productivity
- 2.4 Disturbance
- 2.5 Management and Mitigation
- 2.6 Forest Management Challenges and Opportunities
- 2.7 Forest Adaptation and Management
- 2.8 Urban Forests and Climate
- 2.9 Traceable Accounts
- 2.10 Acknowledgements
- 2.11 Resources
- 2.12 References
2.1 Key Messages
- Climate change is beginning to shift growing conditions for forests in Vermont, with greater changes expected to come, becoming more favorable for southern-adapted tree species and less favorable for currently adapted tree species. Species that will benefit from this change include northern red oak, shagbark hickory, and black cherry, while species including sugar maple, balsam fir, yellow birch, and black ash will be negatively impacted. While growing conditions will be significantly different by 2100, actual change in forest makeup will follow a delay as older trees die and are replaced by young ones.
- Forest productivity, an important indicator of forest health and carbon storage, is amplified by a longer growing season and greater atmospheric carbon dioxide (CO2) and is expected to increase in Vermont in the next 50–100 years. However, productivity will be highly variable by species and will likely begin to decrease by the end of the century as high summer temperatures, drought, and soil nutrient loss outweigh benefits.
- Climate change is expected to continue exacerbating the threats that invasive plants, insects, and diseases already pose to the health of Vermont’s forests. These threats are compounded by other climate-related factors, such as worsening storms and increasingly irregular precipitation.
- Warmer winters and wetter summers already limit active forest management by shortening the time frames that forest operations can take place. These negative climate impacts are projected to strengthen in the future, potentially leading to cascading negative effects on rural economies, forest product markets, and management for forest health and climate adaptation.
- Land use change and parcelization, most commonly conversion of forests to residential or commercial use, is a persistent trend in Vermont, a major threat to forest health and productivity, and a contributor to climate change.
- As climate change impacts forest ecosystem function, there is a need for management to increase forest adaptive capacity. Current methods to achieve increased adaptive capacity at the ecosystem level (retaining ecosystem function despite threats to individual tree species or forest types) include increasing forest structural complexity and enhancing compositional and functional diversity and redundancy.
- Climate change impacts will be more severe for urban trees because of the effects of the built environment on temperature and water cycling, as well as additional stressors associated with urbanized areas like soil compaction, soil fertility, and pollution.
- Urban trees will be increasingly important to humans because of the services they provide. While urbanized areas in Vermont make up less than 2% of the state’s land area, they are home to nearly 243,000 people, 39% of the Vermont population. Because of the high population density and lower tree cover in urbanized areas, per-tree ecosystem services can be higher than in a forest setting. In addition to critical climate and ecosystem benefits provided by trees everywhere, urban trees mitigate the urban heat island effect through cooling and shading and reduce stormwater runoff from extreme rainfall events.
Back to table of contents | See all chapters
2.2 Forest Structure and Composition
To understand the relationship of Vermont’s forests to climate change, it is important to examine the current state of the forests’ structure and composition. Forest structure can be broadly defined by elements such as trees and downed logs and the spatial arrangement of these elements (Franklin et al., 2002). Forest composition describes the number and distribution of species present in a forest. Both influence forest health, function, and resilience to climate change. Vermont’s forest composition is projected to change as climate conditions shift, with warmer-adapted species expanding range across the region and colder-adapted species decreasing range to higher altitudes and latitudes (Iverson et al., 2019). Understanding how the existing forest will respond to climate change is essential to managing it and will impact objectives including conservation, recreation, and aesthetic benefits.
2.2.1 Historic and Current Forest Structure in Vermont
Vermont’s forests have undergone several major shifts before developing into the largely northern hardwood-dominated forests found across much of the state today (Figure 2-1). The most recent drastic change was precipitated by European colonialism. Prior to European settlement, Vermont’s forests were characterized by maple species and beech, with an increasing spruce-fir component further north (Cogbill et al., 2002). Although Indigenous communities utilized the forest, the dominant disturbances were small-scale weather events such as wind and ice storms (Seymour, 2005). For several centuries, land clearing for agriculture, building, and logging increased in Vermont until only 20% of the state remained forested in the late 1800s (Jeon et al., 2014).
During the 1900s, as agricultural production declined in Vermont in favor of more profitable lands elsewhere, forest cover reversed course and began increasing. This agricultural abandonment led to secondary forests (forests growing on previously cleared land) with an even-aged dominance of 80–100 years for most trees, minimal old growth, and some younger age classes arising (Figure 2-2). More recently, younger age classes face establishment challenges including deer browse and less-than-optimal regeneration conditions due to invasive species, as discussed in the Disturbance section. Currently, forests cover 74% of Vermont, a slight decline from 78% near the turn of the twenty-first century. About 80% of Vermont’s forests are owned by families and individual landowners; the remaining is mostly publicly owned, except for a small percentage under corporate ownership (USDA Forest Service, 2020). The most prevalent forest type in Vermont is northern hardwood, and the most prevalent overstory species is sugar maple (Acer saccharum). As climate change shifts many species’ habitability ranges and intensifies disturbances, Vermont’s forests may look as different 100 years from now as they did 100 years ago.
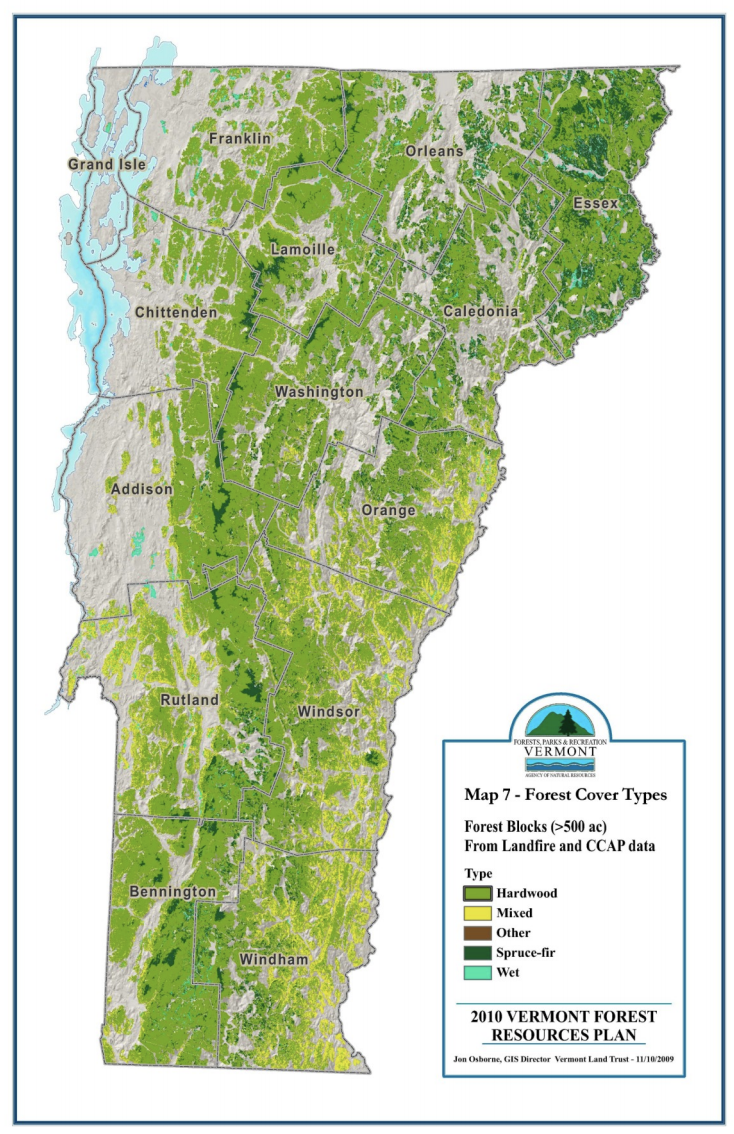
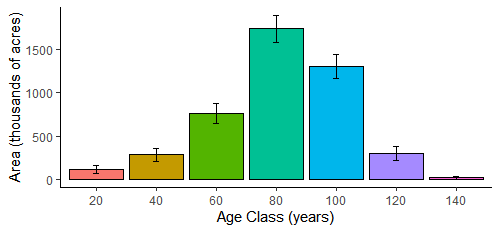
Note: Labels represent the upper limit of each age class; cohort ages are 1-20, 21-40, etc. Black bars represent sampling error at the 95% confidence interval (Morin, 2018, EVALIDator Version 1.8.0.01, 2021)
Forest resilience to climate change is tied to structural, compositional, and functional complexity at both the stand (local) and landscape scale. Within a forest stand, structural complexity relates to the arrangement of mixed elements, such as live and dead trees and downed logs; a structurally complex forest has a varied arrangement of these elements (Figure 2-3A). Landscape-scale structural heterogeneity (diversity of arrangement of structure types, including canopy gaps or groups of old trees) creates a variety of recovery pathways for forests and thus increases forests’ ability to bounce back after a disturbance (Figure 2-3B). Compositional complexity and functional complexity are highly related. A compositionally complex forest has different species present in varying proportions. A functionally complex forest has trees that encompass a variety of functional traits as well as redundancy of these traits across the tree species present. Functional traits are related to plant colonization, survival, growth, and mortality, and they are strongly linked to ecological function (Violle et al., 2007). While species composition can provide information about diversity, it cannot provide specific information about biological function, ecological services, or occupation of ecological niches, all of which are valuable to understand ecosystem function (Messier et al., 2019). Diverse forests are resilient to a wider range of threats: for example, if a disease affects only a single tree species or a specific age class of trees, forests with greater variety in species or age classes will be less impacted by this disturbance than a simpler forest. Managing forests for structural, compositional, and functional complexity has multiple benefits, including enhanced habitat value, greater adaptive capacity, and carbon storage (D’Amato and Palik, 2020; Keeton, 2006). (See also Forest Adaptation section in this chapter.)
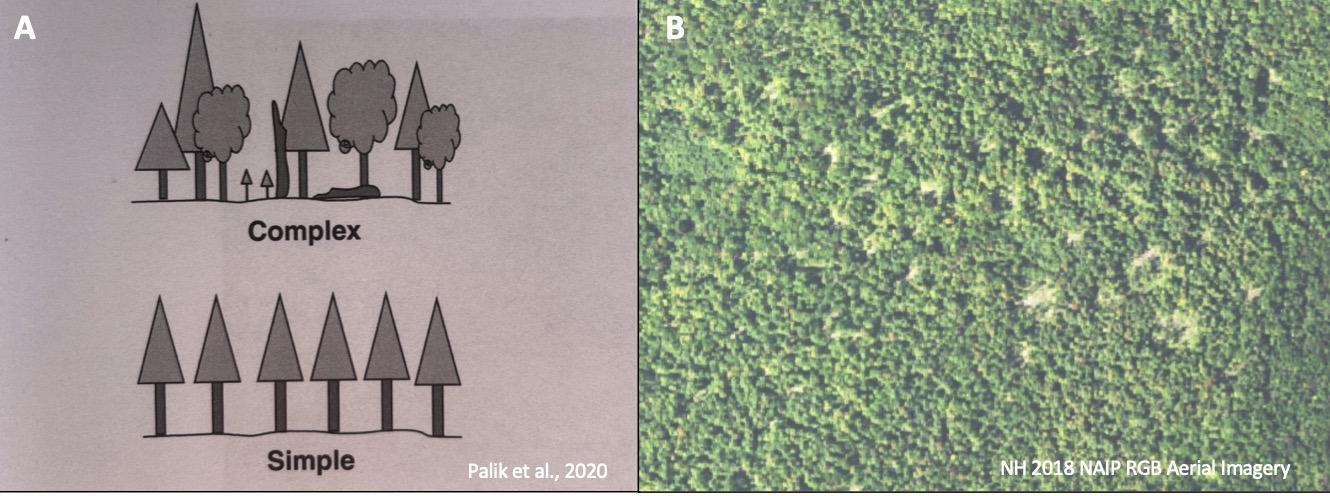
Note: A) A structurally complex vs. simple forest at the stand scale. The complex stand has species diversity, age class diversity, irregular arrangement of trees, and large downed woody material (Palik et al., 2020). B) At a landscape scale, this heterogenous forest contains canopy gaps of multiple sizes, uncut patches of forest in varying sizes, and thinned forest between these elements. Imagery from NH GRANIT (GranitView 2021)
2.2.2 Projected Changes to Forest Composition and Structure
As forest-type zones are predicted to shift northward, Vermont is predicted to become increasingly habitable for oak-hickory forests and less habitable for the northern hardwood and spruce-fir forests that currently dominate the landscape.
The USDA Climate Change Tree Atlas provides comprehensive data and current and projected future ranges for over 100 tree species. Future ranges are calculated using climate models. Each model considers a different set of variables and makes a different set of assumptions, so each model’s predicted future conditions are slightly different. Figure 2-4 compares the current distribution of eastern United States forest types based on on-the-ground forest inventory data, to a potential 2100 distribution, calculated by averaging high-emissions scenarios for three models used to develop the General Circulation Model (Iverson et al., 2019; Peters et al., 2020). Figure 2-5 shows ecoregions in Vermont, and Table 2-1 breaks down the projected changes by tree species and ecoregion in the state.
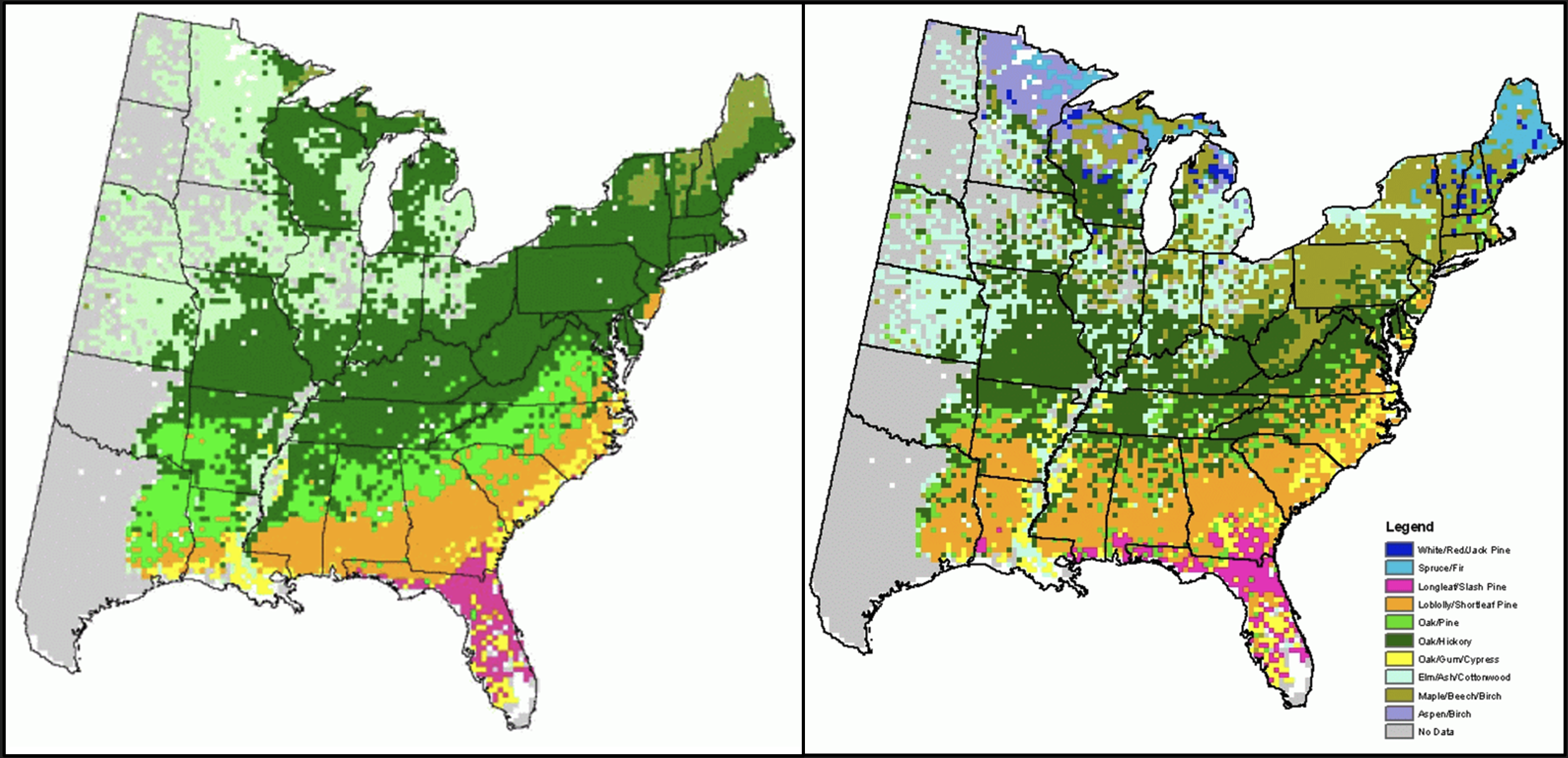
Note: The potential future distribution for the year 2100 is the result of averaging high-emissions scenarios of the three climate models making up the General Circulation Model (Peters et al., 2020).
Table 2-1: Projected species future range shifts in USDA EcoMap regions of Vermont
Under medium-emissions (RCP4.5) or high-emissions (RCP8.5) scenarios, data from the Climate Change Tree Atlas predicts each species’ suitable habitat range to increase, decrease, stay the same, or expand into the region. Modeled scenarios represent the future predicted conditions resulting from different concentrations of greenhouse gas emissions (Iverson et al., 2019; Peters et al., 2020). Adapted from (Catanzaro et al., 2016).
| White Mountains | New England Piedmont | Green Mountains | Champlain Valley | Hudson Valley | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | RCP4.5 | RCP8.5 | RCP4.5 | RCP8.5 | RCP4.5 | RCP8.5 | RCP4.5 | RCP8.5 | RCP4.5 | RCP8.5 |
| balsam fir | decrease | decrease | decrease | decrease | decrease | decrease | same | same | decrease | decrease |
| black spruce | same | same | same | decrease | decrease | decrease | decrease | decrease | decrease | decrease |
| northern white cedar | decrease | decrease | decrease | decrease | decrease | decrease | same | same | decrease | decrease |
| paper birch | decrease | decrease | decrease | decrease | decrease | decrease | increase | increase | decrease | decrease |
| red spruce | decrease | decrease | decrease | decrease | decrease | decrease | decrease | decrease | decrease | decrease |
| tamarack | decrease | decrease | decrease | decrease | decrease | decrease | same | same | decrease | decrease |
| white spruce | same | increase | decrease | decrease | same | same | decrease | decrease | decrease | decrease |
| american beech | decrease | decrease | decrease | decrease | decrease | decrease | same | same | decrease | decrease |
| quaking aspen | increase | increase | increase | increase | increase | increase | same | same | decrease | decrease |
| sugar maple | same | same | decrease | decrease | decrease | decrease | same | decrease | same | decrease |
| yellow birch | decrease | decrease | same | decrease | decrease | decrease | decrease | decrease | same | same |
| bigtooth aspen | increase | increase | increase | increase | increase | increase | increase | same | decrease | decrease |
| eastern white pine | increase | increase | decrease | decrease | increase | increase | decrease | decrease | decrease | decrease |
| red maple | increase | increase | same | same | increase | same | same | decrease | same | decrease |
| american basswood | decrease | decrease | decrease | decrease | decrease | decrease | same | same | decrease | decrease |
| bitternut hickory | expand | expand | same | same | same | same | decrease | same | same | increase |
| black cherry | increase | increase | increase | increase | increase | increase | increase | same | increase | same |
| pitch pine | expand | expand | increase | increase | increase | increase | same | increase | decrease | decrease |
| black birch | decrease | decrease | decrease | decrease | decrease | decrease | same | same | decrease | decrease |
| black oak | expand | expand | increase | increase | increase | increase | increase | increase | increase | increase |
| chestnut oak | expand | expand | increase | increase | increase | increase | increase | increase | increase | increase |
| northern red oak | increase | increase | increase | increase | increase | increase | increase | increase | same | same |
| shagbark hickory | expand | expand | increase | increase | increase | increase | increase | increase | same | same |
| white oak | expand | expand | increase | increase | increase | increase | increase | increase | increase | increase |
| black ash | increase | increase | decrease | decrease | decrease | decrease | decrease | decrease | decrease | decrease |
| eastern hemlock | increase | increase | decrease | decrease | same | decrease | decrease | decrease | decrease | decrease |
| white ash | increase | increase | increase | increase | same | same | same | same | same | same |
Box 2.1: Vermont Sugar Maple
Sugar maple is Vermont’s most common tree species. In addition to the usual tree benefits of shade and wood, its sweet sap plays a big role in the state. Maple syrup production is a major industry in Vermont, representing $54 million in revenue for sugar makers in 2019 (Vermont Agency of Agriculture, Food & Markets 2019 Legislative Summary, 2020). Because sap collection requires specific weather conditions of cold spring nights and above-freezing days, the changing climate may significantly impact maple syrup production in Vermont.
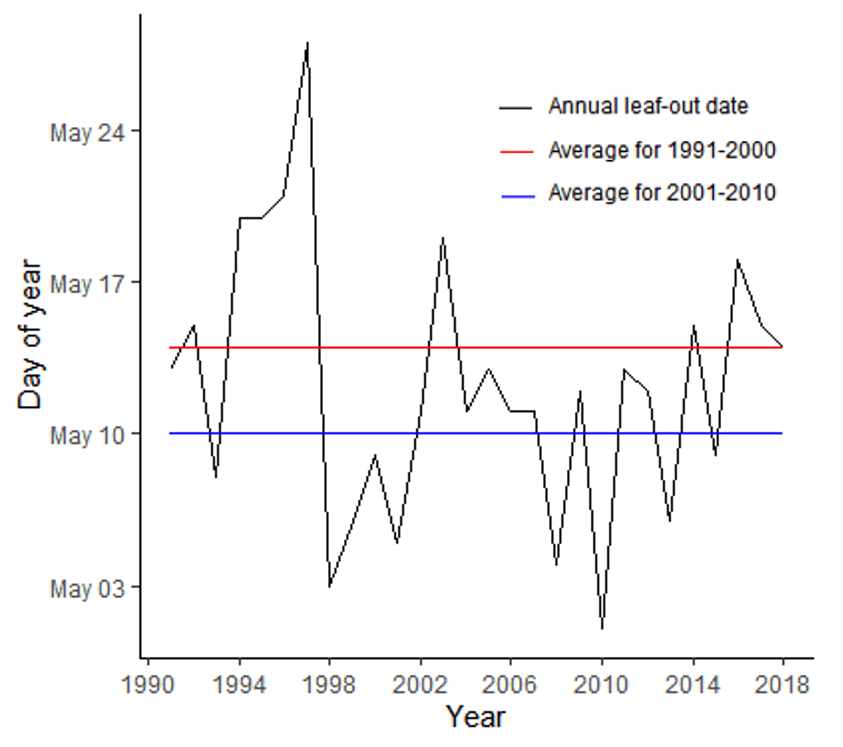
Future climate regime models predict that the habitable area for sugar maple will decrease overall, though some new habitat refuges for sugar maples will become available (Rapp et al., 2019). Climate change may also impact the forest tent caterpillar (FTC), a native insect that eats the leaves of sugar maple and other trees in Vermont. FTC outbreaks occur in periodic cycles, most recently in 2016-2018 (Vermont Department of Forests, Parks & Recreation, 2018). Shifting temperatures are likely to affect FTC survival and shift the synchrony between egg hatching and bud break, though it is not yet clear whether the net effect for sugar maples will be positive or negative (Uelmen et al., 2016). These hungry caterpillars can compound with climate change factors—such as earlier leaf-out dates, late spring frosts, and drought—to increase stress on sugar maples and make them less suitable for tapping (Oswald et al., 2018). In other words, trees already stressed by insect attacks are more vulnerable to the effects of unusual temperature and precipitation changes. Figure 2-6 shows the variance of average leaf-out dates of sugar maples over time; this is just one metric showing how unusual temperature and precipitation changes are influencing sugar maples.
The dates of highest sap flow are shrinking overall and shifting earlier in the season, requiring maple producers to change their schedules to keep up with current levels of production (Guilbert et al., 2014). In the future, even more limited dates of highest sap flow are projected, suggesting that a decrease in syrup production is likely unavoidable. Lower sugar content in sap is another change projected to intensify with the warming climate (Rapp et al., 2019). This means more sap will be required to produce each gallon of syrup, decreasing overall syrup yield for sugar makers.
In summary, direct climate impacts and increased stressors will likely negatively impact one of Vermont’s leading industries. Surveys indicate that most maple producers already are aware of and adapting—or planning to adapt—to the effects of climate change, including by adopting new technologies and shifting where they obtain sap if current pathways become unreliable (Kuehn et al., 2017).
Back to table of contents | See all chapters
2.3 Forest Productivity
Forest productivity is a measure of how much and how quickly a forest grows over time. It is typically quantified as net primary productivity (NPP) in the unit of mass per unit area per unit time. “Net” represents the amount of productivity when taking into account losses, such as from respiration, and “primary” refers to photosynthetic producers in an ecosystem. Net ecosystem production is the physical biomass produced by plants in a forest through photosynthesis, which is mainly composed of carbon. A productive forest takes in carbon from the atmosphere and sequesters (captures from the atmosphere and stores) carbon in the molecules of leaves, branches, and roots. As such, productive forests can buffer the effects of climate change by sequestering more carbon from the atmosphere (IPCC, 2014). Much of this carbon is transferred to fungal partners associated with tree roots, making forest soils a major carbon sink and accounting for approximately 50% of Vermont’s forest carbon (Kosiba, 2021a; Steidinger et al., 2019). Productive forests also typically provide ecosystem services such as water filtration and storm protection, protect themselves against disturbances such as pest infestations and severe weather events, and more quickly recover from such disturbances.
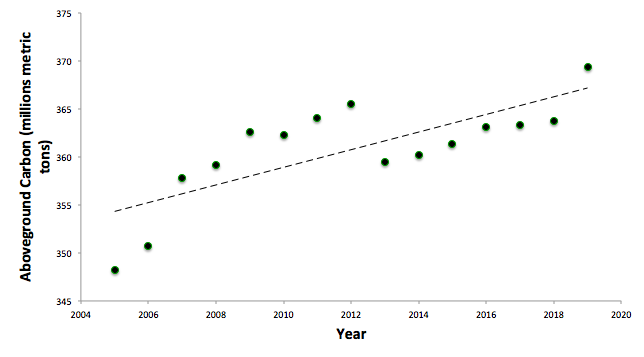
There is moderate evidence suggesting that climate change will increase the carbon stored in Vermont’s forests in the next fifty years (Duveneck and Thompson, 2017; Janowiak et al., 2018) based on both computer modeling and empirical observations (Figure 2-7). On a finer scale, however, the effects of climate change on productivity are likely to vary both spatially and between species due to a combination of indirect climate effects (such as those that may alter soil microbial activity) and different trees’ levels of resilience and adaptability (to drought or extreme heat tolerance, for example). Table 2-2 describes confounding effects of changes caused by climate change.
The productivity of Vermont’s forests is influenced by management practices as well as climate change effects. While forests already store carbon, it may be possible to manage some forested areas for enhanced carbon storage, a desirable outcome considering emerging carbon markets. Carbon storage is just one of many desired management outcomes and must be balanced with needs relating to recreation, wildlife habitat, and economics, among others. (See also the forestry management section in this chapter).
2.3.1 Change in Length and Temperature of the Growing Season
Climate change, particularly warmer winter temperatures, is expected to increase the length of the growing season (Figure 2-8), which has already lengthened by three weeks since 1900 (see Climate Change in Vermont chapter). A longer growing season supports optimal conditions for photosynthesis for more days of the year. In one study of northeastern forests, a 1% increase in the growing season length resulted in a 1.6% increase in net ecosystem productivity (McMahon et al., 2010), with gains mainly in increased aboveground biomass. Interactions are likely to be more complex. Like animals, plants respire (breathe out), using carbohydrate sugars created through photosynthesis to produce energy. Respiration is positively correlated with temperature, so increased summer temperatures may cause increased respiration and may cause decreasing NPP in forests. However, gains in spring productivity are expected to exceed the increased summer respiration (Buermann et al., 2013; Duveneck et al., 2016; Keenan et al., 2014). Respiration may exceed productivity in more extreme warming climate scenarios (e.g., RCP8.5) and/or for tree species that have low temperature optimums for photosynthesis (such as spruce) (Ollinger et al., 2008), although some young trees are able to adjust their physiology to photosynthesize more efficiently under warmer temperatures. In addition to respiration from plants, warmer temperatures have been shown to increase soil respiration from bacteria (Campbell et al., 2009), releasing some soil carbon and decreasing ecosystem productivity overall. By one estimate, temperate forest systems respire 10% of their total carbon, with that number increasing annually (Zhao et al., 2017). Another potentially damaging effect of extended growing seasons is warm spring temperatures that lead to earlier leaf-out may result in frost injury to trees, diminishing the benefit caused by the longer growing season (Hufkens et al., 2012).
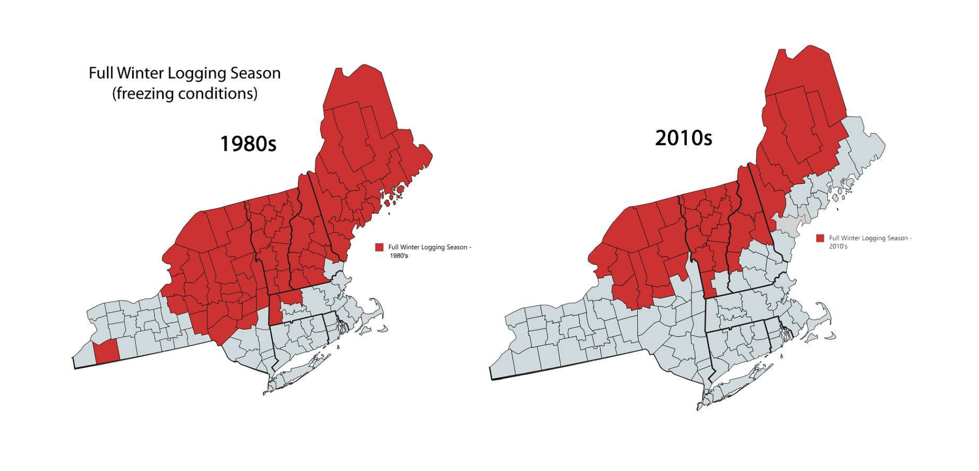
Figure 2-8: Length of Vermont’s Freeze-Free Period by Decade
2.3.2 CO2 Fertilization Effect
“CO2 fertilization” is a phenomenon in which increased levels of atmospheric CO2 enhance photosynthesis rates, thereby increasing tree and plant NPP. Globally, there is potential for forests to sequester more CO2 and effectively lower atmospheric CO2 concentrations (Figure 2-9). Studies have found that elevated atmospheric CO2 concentrations can increase the optimum temperature for photosynthesis in some species and increase plants’ water use efficiency, promising signs that forests may become even more productive and efficient in water use, at least through the next fifty years (Ollinger et al., 2008; Rayback et al., 2020; Sperlich et al., 2020).
While climate change may increase productivity via CO2 fertilization, these benefits may be offset by warming-induced water and nutrient stress (Norby et al., 2010). Additionally, disturbances that interact with climate change—such as fire, insect infestations and increased herbivore populations—will decrease productivity (Couture et al., 2015). The net effect depends on the interactions among atmospheric CO2 concentrations, the sensitivity of tree species to heat, drought and nutrient stress, and external disturbances. For example, the extent to which CO2 fertilization enhances the productivity of spruce forests is limited by their sensitivity to temperature increases. To an extent, the higher NPP and water use efficiency resulting from increased CO2 may allow trees to be nominally more resilient to disturbances brought on by climate change, although more research is needed to determine this.
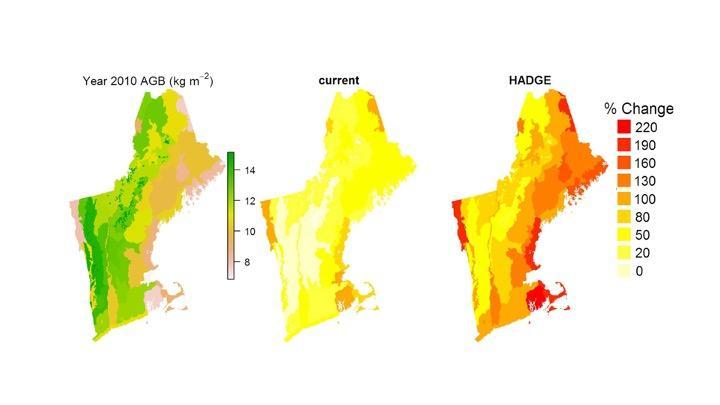
Note: HADGE = Hadley global environmental model v2 – earth system (modified from Duveneck et al., 2016)
2.3.3 Impacts to Nutrient Cycling
To carry out their metabolic processes and produce biomass, trees and other plants rely on nutrients and water from soils. Two of the most important nutrients are phosphorus and nitrogen. Phosphorus is deposited in soils through the weathering of rocks. Nitrogen is abundant in the atmosphere, but most plants can use it only if it is converted to a compound called nitrate (NO3) by nitrogen-fixing bacteria that live in soils and the roots of some leguminous plants. Nitrogen is generally a growth-limiting nutrient in temperate forest ecosystems because nitrate occurs in low concentrations (Campbell et al., 2009).
Warming spring temperatures cause snowpack to disappear earlier in Vermont, impacting nutrient cycling in different ways. Snowmelt may leach nutrients from the soil when it occurs before photosynthesis has resumed and plants can retain nutrients (Contosta et al., 2017; Groffman et al., 2012). Earlier peak snowmelt may limit water availability during the height of the growing season (Wilson et al., 2020), depending on spring and summer rainfall. A combination of earlier snowmelt and decreased snowpack depth (see Climate Change in Vermont chapter) exposes soils to colder temperatures since snowpack tends to insulate soils. Soil-freezing events damage plants’ root tissues so they take up less nitrogen, leading to leaching of nitrogen from the soil over time (Campbell et al., 2014). Under a changing climate, temperature, precipitation, and biogeochemical cycles will interact in new ways that is highly likely to cause a net decrease in key nutrients in Vermont’s soils, limiting the growth of forests and forest productivity
2.3.4 Impact to Beneficial Plant-Fungus Relationships
Soil microbes and fungi play a large role in the productivity of forest ecosystems. Ectomycorrhizal (which surround plant roots) and arbuscular mycorrhizal (which penetrate plant roots) are two of the most abundant root-colonizing fungi that provide trees with critical nutrients and, in exchange, receive carbon from tree roots. These fungi are crucial to carbon sequestration and storage in forest ecosystems.
Certain types of fungal symbiotic partners, in particular ectomycorrhizal fungi, dominate in forests where seasonally cold and sometimes dry conditions inhibit decomposition and where nitrogen is a limiting nutrient—as in Vermont. These fungi have evolved symbiotic relationships with 60% of all tree species, mainly in higher latitudes and altitudes (Steidinger et al., 2019). However, as environmental conditions become more like lower latitudes and altitudes (e.g., warmer, wetter), these fungi are threatened. Declines in such fungi could cause declines in the trees adapted to them, including beech (Fagus grandifolia), white pine (Pinus strobus), oaks (Fagus spp.), and many other northern hardwood species. However, when combined with other climate adaptability indicators of tree species, different patterns emerge. For example, the range of oak species may expand (Figure 2-4, Table 2-1). Change in fungal composition in Vermont’s soils along with increasing temperature and precipitation will facilitate changes in the composition of forests (Classen et al., 2015). Changing temperature and precipitation regimes are also compromising the functionality of the fungi and microbes, which trees depend on to assimilate nutrients from the soil, causing negative impacts on productivity that may not be mitigated by longer growing season length and CO2 fertilization.
2.3.5 Atmospheric Deposition
Humans release many compounds that can affect forest productivity to the atmosphere. For example, volatile sulfur and nitrogen compounds originating from industrial activities are deposited from the atmosphere by rain and snowfall in relatively high concentrations in the northeastern United States (“atmospheric deposition”) (Pardo et al., 2011), though the production of these compounds is expected to decrease because of measures to reduce greenhouse gas emissions. Atmospheric deposition of nitrogen and sulfur to soils can alter the biogeochemistry of forest ecosystems. Nitrogen saturation has been shown to decrease the allocation of carbon to mycorrhizal fungi, weakening the essential relationship between trees and fungi (Frey et al., 2014). Nitrogen and sulfur deposition has been shown to make trees more vulnerable to drought and insect infestations, weakening resilience to climate change (McNulty and Boggs, 2010; Pardo et al., 2011), and acid deposition can leach nutrients from foliage and forest soils, leading to nutrient depletion (DeHayes et al., 1999). While atmospheric deposition is not a direct effect of climate change, it comes from the same root cause (e.g., human combustion of fossil fuels). The use of products containing nitrogen and sulfur increased for many decades before regulations began limiting production. There are indications of ecosystem recovery from some of these pollutants (Kosiba et al., 2018) but stress brought on by climate change could exacerbate the negative effects of nitrogen and sulfur deposition.
Ground-level ozone is an atmospheric pollutant that affects stomatal control, decreases water use efficiency, and damages plant tissues, thus causing water stress and decreased biomass and productivity. Plants with increased ozone exposure have declines that offset gains in productivity from CO2 fertilization (Mohan et al., 2009; Rustad et al., 2012) Ozone levels are closely linked to the presence of nitrogen oxides (products of fossil fuel combustion) in the atmosphere, so decreased ozone emissions could have positive effects for forest productivity barring negative interactions with other climate change stressors.
Table 2-2: Factors interacting with climate change and affecting productivity for forest ecosystems in the Northeast
| Confounding factor | Effect |
|---|---|
| Nutrient availability | Less fertile soils, characterized by declines in essential nutrients such as nitrogen and phosphorus, are expected due to a combination of increased 1) decomposition rates, 2) leaching of soil nutrients due to earlier snowmelt and increased precipitation events, and 3) uptake rates by plants with enhanced metabolisms due to CO2 fertilization. |
| Water availability | While Vermont has experienced increased annual precipitation, there are two factors that could lead to water limitation: 1) Earlier onset of snowmelt, if not mitigated through rainfall, will lead to lower water table levels. 2) Warmer temperatures will increase rates of evapotranspiration from plants and lead to a decrease in water use efficiency. |
| Heat stress | Tree species that are intolerant to intense heat, such as spruce, may experience declines in productivity in the summer. |
| Biotic stressors | Climate change is expected to increase the prevalence of pests and pathogens (see the disturbance section in this chapter), invasive species, and herbivores (such as white-tailed deer) due mainly to milder winters. The range and fecundity of these species will expand and become more established in Vermont. Most tree types will experience direct decreases in biomass and productivity due to biotic stressors. |
| Arrested succession | Both native and invasive plant species can form dense, monodominant understories. Such understories can form quickly after disturbances, particularly where excessive deer grazing favors the proliferation of less-desired browse species. Dense understories can limit forest regeneration, outcompeting slower growing trees for physical space, nutrients, and sunlight, and thus arresting forest succession. |
| Atmospheric deposition and acid rain | Acid deposition in the Northeast originates from industrial activities producing nitric or sulfuric oxide emissions that combine with atmospheric water vapor to form acid rain. Acid deposition could increase with increased precipitation events, though high levels of water vapor may also dilute effects, or emissions may be reduced through emissions reductions. The impacts of atmospheric deposition and acid rain on nutrient availability will especially affect high-elevation spruce/fir forests, which are already suffering from heat stress. |
Back to table of contents | See all chapters
2.4 Disturbance
Ecological disturbance (e.g., high winds, fire) plays a major role in forest structure and function. Climate change may compound the effects of biotic disturbances such as invasive forest pests, plants, and pathogens. Simultaneously, climate change is projected to increase the frequency and severity of abiotic stressors, such as extreme weather events (Reidmiller et al., 2018). Forests are constantly responding to minor disturbances, but the expanding frequency and intensity of these stressors is unprecedented in recent times. In addition, disturbances may interact; for example, more frequent stand-replacing weather events may provide better habitat for shade-intolerant invasive plants that can aggressively displace native species (Dukes et al., 2009).
2.4.1 Biotic Disturbances
Forest pests and pathogens, herbivores, and invasive plants are all agents of disturbance in Vermont’s forests, and they intersect with climate change to varying degrees. Of the many exotic species brought to the United States intentionally or unintentionally each year, a fraction are considered invasive due to their abilities to aggressively spread, kill, or displace native species. Non-native insects and diseases may functionally eliminate a tree species or group of species, such as ashes or hemlocks, significantly altering the forests in their invasive range (Lovett et al., 2016). Others, such as a group of fungal pathogens known as anthracnose disease, inflict low-level stress on a variety of species, contributing in conjunction with other factors such as drought or changing climate suitability to overall forest decline. Although effects vary among species, in many cases warming trends enable invasive species to spread faster by decreasing winter mortality and increasing reproduction rates (Seidl et al., 2014). Table 2-3 summarizes the impacts and climate interactions of several non-native pests and pathogens of concern in Vermont, including some that are already established in the state and others that may arrive soon.
Invasive plant species represent a different threat to Vermont’s forests. Instead of directly killing trees, they out-compete native plants, including many understory species, and take up sunlight and other resources. Although invasive plants are found in different habitats and conditions, they are often shade-intolerant and spread or reproduce prolifically. Increasing forest fragmentation and human-caused disturbances, along with native species that are more vulnerable due to climate change, lead to increased dominance by invasive plants. Invasive plants displacing native plants have ripple effects through the ecosystem: they remove food sources for wildlife and alter soil chemistry, nutrient cycling, and water cycling (Fisichelli et al., 2014).
Repeated disturbances often lead to reduced diversity and proliferation of dense understories that prevent or delay the natural succession of forests; these dense, monodominant patches are called recalcitrant understories. Recalcitrant understories are usually composed of fast-growing and browse-tolerant species that increase light percolating to the forest floor. They are caused by excessive deer grazing in tandem with outbreaks of diseases or pests, such as the LDD moth (Lymantria dispar) (Royo and Carson, 2006). Fundamentally, canopy disturbance and ground level manipulation are likely to significantly alter the successional trajectory of forests. Causes—including deer browse and disease outbreaks—are expected to increase in frequency and severity in Vermont because of climate change (see Fish and Wildlife in Vermont chapter). Further, pressure from deer browsing may limit the ability of forest ecosystems to respond to climate change (Fisichelli et al., 2012). The outcome for many of the hardwood species valued in Vermont is less vigorous regeneration. Deer browsing combined with other climate stressors could contribute to the decline of forest types in Vermont.
Table 2-3: Invasive pests and pathogens of current or future concern in Vermont forests
| Pest or pathogen | Description | Species impacted | Geography | Climate interaction | References |
|---|---|---|---|---|---|
| Balsam woolly adelgid (Adelges piceae) | Small, aphid-like insect that feeds on trees’ internal tissues, particularly older trees | Balsam fir (Abies balsamea) | Statewide in VT | Populations are limited by temperature extremes, expanded by milder seasons in comparable forests in other regions | Hrinkevich et al., 2016; Quiring et al., 2008 |
| Beech bark disease (Nectria fungi) and beech scale insect (Cryptococcus fagisuga insect) | Combination of at least two fungal infections and sap-feeding scale insects that weaken trees, affecting their ecological values | American beech (Fagus grandifolia) | Statewide in VT | Increasing periods of drought make beech more vulnerable to factors like beech bark disease, as beech is less climate resilient than many other hardwoods. Milder winters favor beech scale insect | Stephanson and Coe, 2017 |
| Earthworms | Variety of worm species originating in Europe accelerate litter decomposition, altering forest floor structure | Many overstory and understory species | Statewide in VT | Overstory and understory species are impacted by the changes worms make on the soil profile, which affects regeneration of species and moisture retention in the soil. It is likely to amplify other climate effects like changing precipitation regimes and soil communities | Dobson and Blossey, 2015 |
| LDD moth (Lymantria dispar dispar) | Moth whose caterpillar feeds on leaves of many trees, with rapid population growth occurring in irregular outbreaks; consecutive years of defoliation can kill trees | Oaks (Quercus spp.), apples (Malus spp.), maples (Acer spp.), birches (Betula spp.), and many other species | Statewide in VT | Drought leads to LDD moth outbreaks because it limits the growth of a fungus that kills and weakens LDD. Climate change is likely to increase the variability and extremity of weather patterns, including extended periods of drought that could lead to more frequent LDD outbreaks & more severe impacts in the future | Davidson et al., 1999 |
| Anthracnose disease | Various species of fungus causing leaf damage that weakens and rarely kills trees | Maples (Acer spp.), ashes (Fraxinus spp.), oaks (Quercus spp.), sycamores (Platanus spp.), dogwoods (Cornus spp.), and others | Several locations across VT | Thrives in moist conditions, so changing precipitation regimes may affect its spread, frequency, and severity; susceptible tree species may increase or decrease range in Vermont due to climate change | Holzmueller et al., 2010; Vermont Forest Health, 2011 |
| Elongate hemlock scale (Fiorinia externa) | Scale insect that feeds on needles, stressing trees in combination with hemlock woolly adelgid | Eastern hemlock (Tsuga canadensis) | Several locations across VT | Demonstrated ability to adapt locally to different temperatures may enable its continued spread throughout hemlock’s range | Preisser et al., 2008 |
| Emerald ash borer (EAB) (Agrilus planipennis) | Green beetle feeding on inner bark and sapwood in its larval stage, weakening and usually killing trees | Ashes (Fraxinus spp.) | Several locations across VT | Ash and EAB ranges are predicted to shift under climate change; phenological mismatch between EAB and biocontrol agents may occur | Jones et al., 2020; Liang and Fei, 2014 |
| Hemlock woolly adelgid (Adelges tsugae) | Small, aphid-like insect that feeds on trees’ stored starches | Eastern hemlock (Tsuga canadensis) | Windham, Bennington, and Windsor counties as of 2019 | Currently restricted to southern VT due to cold winter temperatures, but warming winters may expand range northward | McAvoy et al., 2017 |
| Sirex woodwasp (Sirex noctilio) | Large, wood-boring insect often transported through wood packaging and firewood | Pines (Pinus spp.) | Present in VT (detected in Lamoille County as of 2008) | Trees stressed by changing climate factors (e.g., increased drought) are more vulnerable to infestation by this pest | Slippers et al., 2011 |
| Asian longhorned beetle (Anoplophora glabripennis) | Large black and white-spotted beetle that feeds on wood in its larval stage, weakening host trees | Hardwood species including maples (Acer spp.), ashes (Fraxinus spp.), birches (Betula spp.), poplars (Populus spp.), and more | Not detected in VT; present in MA and NY | High temperatures and precipitation limit Asian longhorned beetle dispersal, but effects are not consistent across its range | Huang et al., 2020 |
| Spotted lanternfly (Lycorma delicatula) | Leafhopper insect feeds on sap, stressing and sometimes killing trees | Maples (Acer spp.), birches (Betula spp.), grapevines (Vitis spp.), and many other species | Not detected in VT; present in NY, CT, PA, and NJ | Often co-occurs with tree-of-heaven, its preferred host species, and an aggressive invasive plant | Urban, 2020 |
| Winter moth (Operophtera brumata) | Caterpillar that feeds on buds and new leaves in spring | Oaks (Quercus spp.), maples (Acer spp.), birches (Betula spp.), apples (Malus spp.), blueberries (Vaccinium spp.), and others | Not detected in VT; present in MA, RI, NH, ME, CT, and NY | Warmer winters may allow it to expand its range into Vermont; changing weather disrupts winter moth’s synchrony with host tree bud break, some shift to other species | Elkinton et al., 2015 |
Box 2.2: Emerald Ash Borer and Climate Impacts in Vermont
Emerald ash borer (EAB; Figure 2-10) is one of the most destructive invasive forest pests in the United States. This shiny green beetle, native to Asia, feeds on ash tree sapwood and inner bark in its larval life stage, weakening and eventually killing trees. Its spread has caused economic, ecological, and cultural losses as it has killed millions of ash trees in cities and forests (McCullough, 2020).

EAB’s rapid expansion in North America is largely attributed to unintentional human movement through infested firewood. Quarantine regulations helped keep EAB out of Vermont for many years, but it was first detected in Vermont in 2018 (Figure 2-11) and has since spread to several locations (“Emerald Ash Borer (EAB) Infested Area in Vermont,” 2021). Avoiding movement of firewood is still encouraged to slow its spread, but at this point EAB is likely to continue to expand its range throughout Vermont’s forests.
Climate change may alter the dynamics between EAB and ash trees in North America, though these effects are likely to take place on a longer time scale than the current speed of EAB-induced mortality. One study suggests that climate change will exacerbate EAB’s impacts in the northern part of its invasive range, including Vermont, because the future climate will be more favorable to EAB (Liang and Fei, 2014).
While there is no silver bullet that can prevent EAB, there are several options to manage EAB and forests containing ash. They include:
- Chemical control: The pesticide emamectin benzoate is highly effective at protecting individual ash trees from EAB, but its expense and impermanence limits its widespread use. It can be combined with girdling “trap” trees to attract and then kill EAB.
- Biological control: Several EAB parasitoids from its native range have been released in the United States in efforts to reduce pest populations. Though they are not available for individual use, growing populations may contribute to landscape-level EAB control, most often used in conjunction with other methods.
- Genetic resistance: A small proportion of North American ash trees display natural resistance to EAB, and research efforts are underway to identify these individuals, understand their mechanisms of defense, and breed them to create more resistant ash stock.
- Silvicultural strategies: When managing forests with ash for EAB, it is important to keep in mind that diverse forests are resilient forests. Retaining some mature ash in the face of EAB allows resistant individuals to persist and reproduce. The habitable range for white ash in Vermont is predicted to increase, and if it can be preserved to some extent through the immediate threat of EAB, ash may continue to be an ecologically significant component of Vermont’s future forests (D’Amato et al., 2020).

Box 2.3: LDD Moth (Lymantria dispar dispar) Outbreak of 2021
In 2021, Vermont and other parts of New England saw the largest outbreak of the Lymantria dispar (LDD) moth (Figure 2-12) in three decades (Vermont Department of Forests, Parks & Recreation, 2021). LDD is an invasive pest that was introduced to North America in 1869. LDD caterpillars prefer feeding on oak leaves but will eat the leaves of many other trees, including maples, birches, and even pines when their favorite host is not available (Davidson et al., 1999). The caterpillars in 2021 were prolific enough that they caught the notice of many Vermonters (Robinson, 2021). In addition to defoliating many trees, LDD caterpillars can cause uncomfortable rashes for some people who come into contact with them (Kikuchi et al., 2012).
Populations of LDD, though present at low levels each year, are usually kept in check by a fungus that needs wet conditions to thrive. Vermont’s recent drought conditions have prevented the fungus from carrying out its population control, leading to the 2021 LDD explosion. The next few years could see more high levels of LDD if dry periods continue. Though one year of LDD defoliation is not catastrophic for trees (Figure 2-13), two or more consecutive years could be deadly (Vermont Department of Forests, Parks & Recreation, 2021). Especially in combination with other stressors like drought, LDD is a serious threat to Vermont’s forests. Climate change is causing more variable and extreme precipitation changes. If more drought is in the future, then more LDD destruction certainly is too (Davidson et al., 1999). Vermonters should read the advice from the Vermont Department of Forests, Parks, and Recreation to control the spread of LDD in future years (Vermont Department of Forests, Parks & Recreation, 2021).


2.4.2 Abiotic Disturbances
Although large-magnitude disturbances such as windstorms and ice storms are historically rare in Vermont, these extreme events are projected to increase in frequency and severity due to climate change (Reidmiller et al., 2018). Models indicate that such events will occur with increasing stochasticity (unpredictability), making specific future impacts difficult to forecast (Janowiak et al., 2018). Abiotic disturbances have a range of impacts on Vermont’s forests, from tree mortality to altered forest structure to changing soil conditions.
Box 2.4: Effects of Future Precipitation Scenarios on Forest Regeneration
Written by Peter Clark, University of Vermont
Climate change has already increased precipitation and altered the intensity of precipitation in Vermont, trends that are expected to continue (see Climate Change in Vermont chapter). In the northeastern United States, heavy precipitation events (>1 inch per day) punctuated by long periods of drying are already more common and are projected to increase throughout the twenty-first century with broadscale consequences on forest ecosystem function, composition, and the delivery of ecosystem services.
A recent scientific experiment was conducted in the University of Vermont’s Jericho Research Forest to understand the effects of future precipitation changes on forest regeneration (Clark and D’Amato, 2019). This study focused on recently harvested areas that are now forest gaps (Figure 2-14). Within forest gaps, seedling germination and survival was measured with experimental treatments controlling precipitation. Replicated precipitation manipulation experiments were installed for a series of monitoring plots. Experimental treatments manipulated water input to simulate historic precipitation conditions in some plots and scenarios of future precipitation in others. The precipitation manipulations included total precipitation as well as different combinations of rainfall frequency and heavy precipitation events, creating extreme short- and long-term drying and wetting conditions.
This study showed that seedling survival was controlled by precipitation treatment, functional traits (e.g., seed mass), and planting microsite. While the role of climate in seedling survival has been well described in scientific literature (e.g., Fisichelli et al., 2014), this study showed that the survival response to precipitation was largely controlled by species-specific functional attributes. For instance, species with smaller-massed seeds (i.e., birches, pines, maples, and hemlock) were significantly affected by differences in precipitation treatments, but larger massed species (i.e., oaks, hickories, beech, and chestnut) were unaffected.
These findings suggest that heavy precipitation events will not be enough to offset moisture deficits during prolonged dry periods in the future when it comes to seedling germination and survival. Future forest regeneration will favor species adapted to extended drying. Precipitation played an important role in seedling survival, but this study also showed that seedbed microsite conditions (e.g., mixed scarified > unmodified forest soils) were over twice as important in determining seedling survival than the precipitation regimes in the study. The implications of this work suggest that interacting effects of climate, species functional traits, and microsite conditions via disturbance or management will influence many aspects of the regeneration of future northeastern forests.

Back to table of contents | See all chapters
2.5 Management and Mitigation
Vermont’s forests are an important economic and ecological resource: the forest products industry generates approximately $1.4 billion in revenue and supports 10,500 jobs annually (Vermont Sustainable Jobs Fund, 2017). Forest management in Vermont creates jobs, provides essential local wood and non-wood forest products, and protects forest health. Vermont’s forests currently store approximately 1,730 million metric tons of carbon dioxide equivalent (MMt CO2e). Since 1990, Vermont’s forests have removed or sequestered approximately 5.5 MMt CO2e per year from the atmosphere (Kosiba, 2021a).
Forest management occurs along a spectrum ranging from intensive harvesting activities to passive management and not harvesting trees. Decisions along this spectrum seek to meet goals of biodiversity, carbon sequestration and storage, periodic income, timber and non-timber forest product output, recreation, aesthetics, forest health, and global change adaptation. Generally, any management decision requires calculating tradeoffs between the above objectives. Passive management does not necessarily mean that a forest will remain undisturbed in the long term, but it does mean that the next disturbance is less likely to be directly caused by humans (see Disturbance section).
Climate change poses a threat to forest management operations, as shorter, warmer winters make it harder to carry out management activities typically conducted when the ground is frozen. These new challenges have cascading negative effects on rural economies and management actions that would benefit forest climate change adaptation, response to forest disturbances, wildlife habitat, and forest productivity.
Globally, forest management with a primary or solitary goal of carbon storage or sequestration is becoming popular as a climate mitigation solution, but management for a singular goal may have detrimental effects on forest economies, biodiversity, and forest health at the expense of other forest processes and ecosystem services. For example, a forest that is managed passively may have higher carbon stocks (i.e., carbon in aboveground biomass) than a recently harvested forest, but it could be sequestering carbon at a lower rate (i.e., lower NPP) and due to past human activity, lack the structural and functional complexity that provides it resistance or resilience to climate change (e.g., Bradford and D’Amato, 2012). Further, owning land costs money (e.g., landowners must pay taxes, maintenance of access to the land), and periodic timber harvests provide income to pay these expenses, incentivizing landowners to keep forests as forests. Wilderness conservation easements or payments for carbon storage (see Mitigation section) often do not provide the same sort of periodic long-term income (Graves et al., 2020).
2.5.1 Impacts on Length of Logging Season
The logging industry in Vermont historically has been dependent on operations that take place in the winter on frozen ground or on snowpack atop frozen ground, as loggers work to minimize soil erosion and potential damage to tree root systems at sensitive sites. Warmer nighttime temperatures in December and January (see Climate Change in Vermont chapter) mean that the ground is not freezing fully as early as it used to, and it is thawing earlier in the spring, shortening the logging season, (Figure 2-15; Contosta et al., 2019). Vermont’s freeze-free period has increased by three weeks since 1960 (see Climate Change in Vermont chapter). Further, snow often falls before the ground is fully frozen, insulating the ground from frost and potentially leading to winters where the ground never fully freezes (Rittenhouse and Rissman, 2015). These conditions reduce the number of operable days for logging contractors.
Dry summer months offer the second major logging season in the state. However, increased rainfall in June and August, often falling in the form of heavy precipitation events (greater than one inch), saturates the soils with water. This limits loggers’ ability to operate during these months without causing significant ecological damage or physical damage to infrastructure and machinery.
Poor logging conditions also lead to a need for longer contracts; rather than finishing jobs in six months to one year, contracts often need to last two full years to ensure enough operable days. This can disincentivize landowners to take on active management activities and mean that logging contractors must spread their operations across many sites based on weather and market conditions.
2.5.2 Impacts on Logging Occupation
One way that logging contractors improve their ability to operate in poor conditions is to expand or alter their equipment inventory. It often becomes necessary for contractors to own a bulldozer or excavator to protect sites, adding to costs when there is already a low profit margin (Kuloglu et al., 2019). Different logging equipment choices are needed for different sites and conditions, so operators are forced to make tough decisions about what might serve them best in the future. Sustained or higher costs combined with fewer days available to work means that fewer logging contractors can maintain their businesses. Between 2002 and 2016 there was a 36% decrease in jobs in the forest products industry and 11% decrease in forest products businesses (Vermont Sustainable Jobs Fund, 2017). These changes are not solely climate driven, as the 2008 recession played a large role, but highlight a trend that is and will be exacerbated by climate change.
2.5.3 Impacts and Adaptation of Sawmills
As climate change limits timber harvest, it also threatens the ability of sawmills to procure enough wood of sufficient quality in a timely and consistent manner to remain solvent. Reduced operation time for logging contractors and low capacity for timber harvesting mean that sawmills may struggle to bring in enough wood to keep their operational rate consistent. Historically, sawmills were able to stockpile logs through the winter season, leaving them with enough wood to saw during other seasons (Bick et al., 2019). A shorter winter harvest season means a need to store more logs whenever they can be cut, which may be restricted by lack of storage space and the fact that cut logs have a limited period of viability before they must be sawn.
Sawmills may also be restricted by the costs and carbon emissions associated with transport of logs, lumber, pulp, and sawdust. Exacerbating these limitations, forest products are often moved outside of Vermont, and poor road conditions, such as those caused by icy conditions or winter thaws, further increase costs. Even though regional mills are not vertically integrated (generally, they do not own the land that is the source of their logs), they may need to work on investing in on-the-ground operations by supporting suppliers. Sawmill support of logging operations may take the form of funding and lending portable skidder bridges, maintaining flexibility in wood delivery timing, providing support for equipment upgrades, preferentially purchasing wood from operators with equipment that has a lighter touch on the land, and providing financial support for site maintenance activities such as adding gravel and grading roads.
2.5.4 Impacts on Wood Markets
Wood prices, including lumber, are influenced by harvesting conditions, mill operations, and markets. In Vermont, sugar maple and red oak tend to fetch the highest stumpage prices (price paid for standing timber; Figure 2-16). Prices can be volatile, and markets may change suddenly, as evidenced by across-the-board price reductions in 2020 at the start of the COVID-19 pandemic. Demand for wood drives the price that a sawmill can charge for lumber, but site accessibility, sawmill capacity, and weather conditions influence how much a landowner can expect to be paid for their standing timber. This disconnect makes it hard to predict how future prices for both standing timber and processed lumber might change, though limits to site access are likely to reduce the dollar value paid to a landowner. Species like sugar maple and ash tend to grow in sites that are richer and moister and more sensitive to marginal operating conditions, while oak trees often dominate on dry sites. Oaks are at the northern edge of their range in Vermont and are more commonly harvested in the southern part of the state.
Sale of low-grade wood products for pulp, firewood, pellets, and biomass provides source material for essential wood outputs for home heating and paper production and subsidizes forest management operations that improve forest health and timber quality. Price and demand of these low-grade products are dependent on mill capacity, and the closure of a large pulp mill in Maine in 2020 is expected to limit the market for these materials.
Wood prices are variable even absent climate change. Individual tree species fall in and out of popular favor, housing markets fluctuate, and markets for low-grade products change based on fossil fuel prices and policy change (e.g., timber price drop after 2008 recession, price increase under 2017 export tariffs; Figure 2-17). Wood markets in Vermont do not operate in a vacuum; logs are exported, and wood products are imported. A more detailed prediction of future market changes would be a fool’s errand; however, a portfolio approach of managing for healthy trees regardless of species presents a way to buffer against changes in an unknown future.
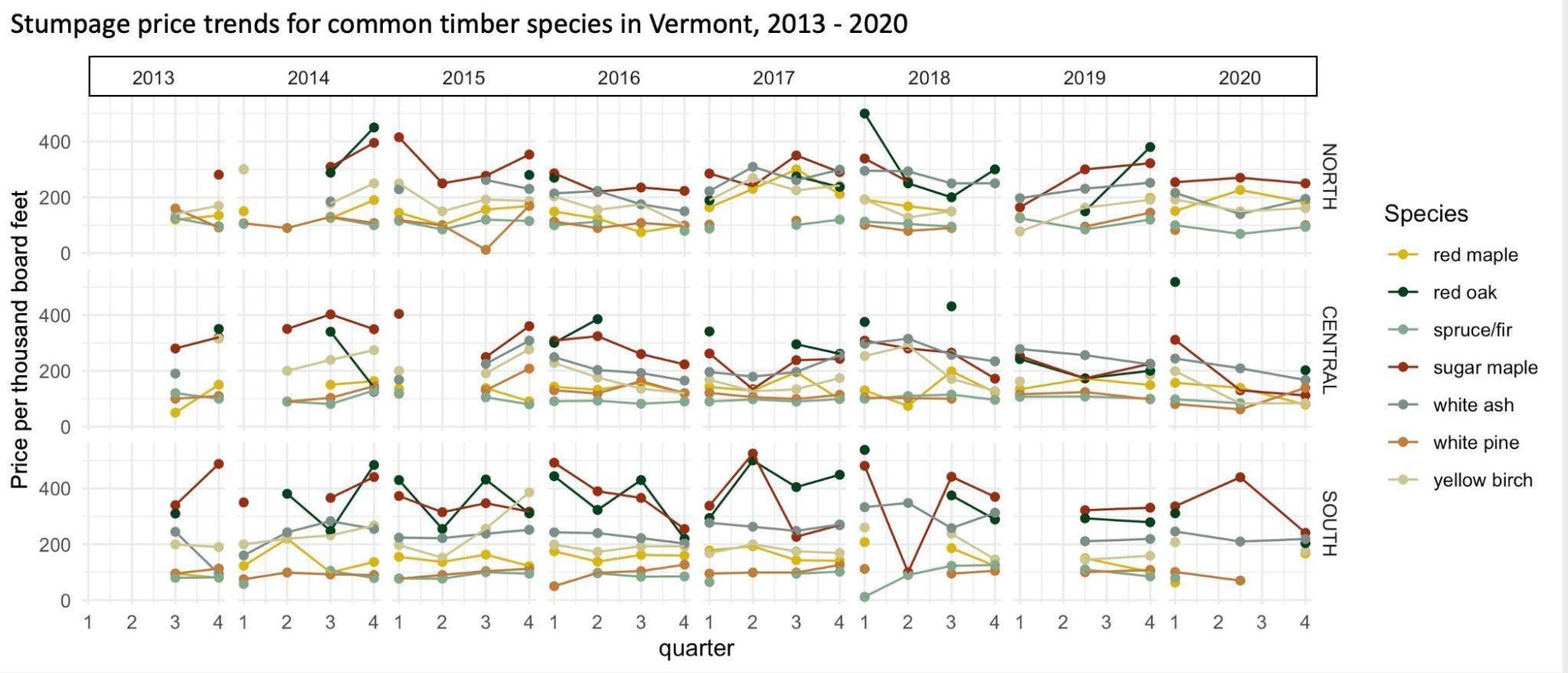
Notes: The values represented here are intended to be indicators of relative stumpage value and used for guidance only, as many are based off one or two sales. They are not statistically valid and meant to only represent general trends. North = Caledonia, Essex, Franklin, Grand Isle, Lamoille, Orleans; Central = Addison, Chittenden, Orange, Washington; South = Bennington, Rutland, Windham, Windsor, Source: VT FPR
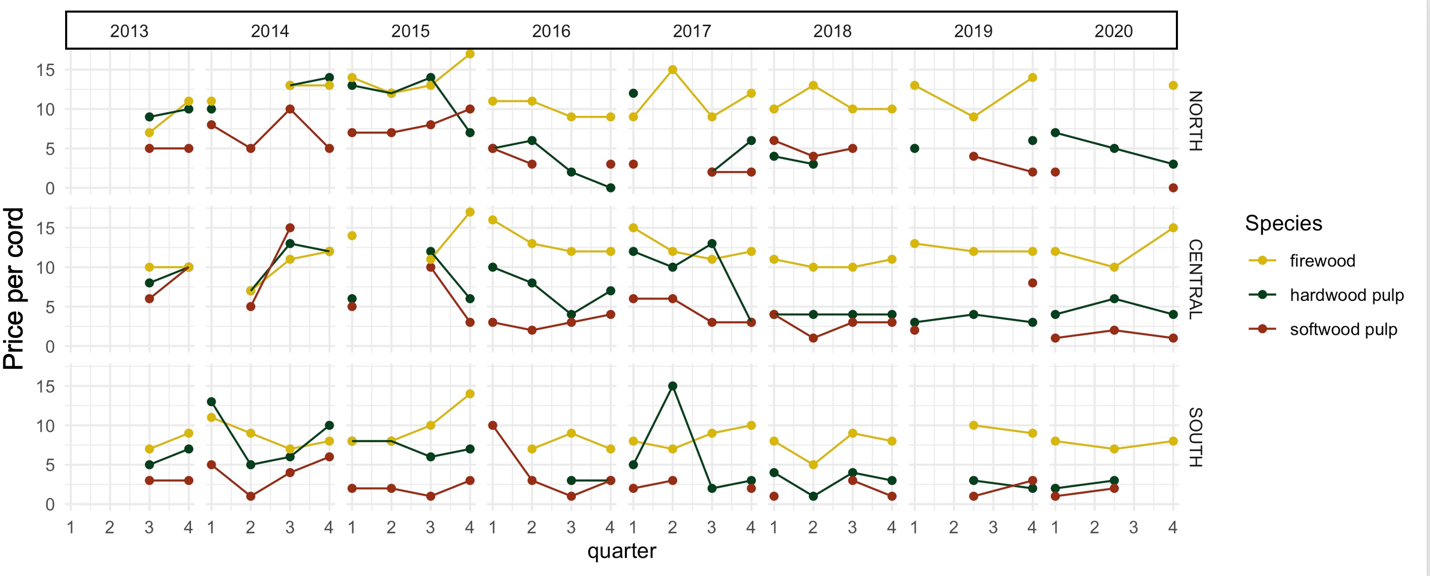
Notes: The values represented here are intended to be indicators of relative stumpage value and used for guidance only, as many are based off one or two sales. They are not statistically valid and meant to only represent general trends. North = Caledonia, Essex, Franklin, Grand Isle, Lamoille, Orleans; Central = Addison, Chittenden, Orange, Washington; South = Bennington, Rutland, Windham, Windsor Source: VT FPR.
2.5.5 Infrastructure Costs
As precipitation and winter temperatures increase, road improvements are often needed for operators to move wood on harvest sites and along roads to mills. On harvest sites, this means more time and resources spent on road construction, increased need for gravel to stabilize roads, and more robust structures to manage water flow. On paved roads where heavy log trucks travel to mills, water flow structures are likely to need improvement to handle increased run-off and avoid damages to roads.
The United States Forest Service has adopted stream-simulation design, or drainage structures that retain the natural stream bottom rather than round culverts, as the preferred approach in their forest road systems. In the Green Mountain National Forest, Vermont identified culverts that were replaced with stream-simulation design before Tropical Storm Irene occurred (Gillespie et al., 2014) The difference in cost between using the prior design and the stream-simulation design varied from 9 to 22%. Even more noteworthy is the difference between the cost of the alternative design (Table 2-4a) and flood damages to culverts like the prior designs that were not replaced before the storm (Table 2-4b): despite higher initial costs to install stream-simulation design drainage, the preferred design avoids a much higher road repair cost after an extreme precipitation event.
Table 2-4: Culvert costs on Green Mountain National Forest (a and b) (Gillespie et al., 2014)
| Estimated costs from damage survey report | |||||
|---|---|---|---|---|---|
| ROAD NO./NAME | TRADITIONAL CULVERT/REPLACE IN KIND ($) | BETTERMENT/AOP STREAM SIMULATION REPLACEMENT ($) | ANTICIPATED % COST INCREASE FOR AOP STREAM SIMULATION DESIGN | ACTUAL CONSTRUCTION COST ($) | ACTUAL % COST INCREASE FOR AOP STREAM SIMULATION DESIGN |
FR42.05.0 over Bingo Road |
92,950.00 | 142,050.00 | 53 | 113,738.00 | 22 |
| FR42B.00.0 over Bingo Brook | 112,175.00 | 156,775.00 | 40 | Never constructed, road decommissioned | NA |
| FR49.00.5 Boyden Brook | 93,800.00 | 140,700.00 | 50 | Never constructed, Irene damaged site access road | NA |
| FR92.00.0 over Goshen Brook | 106,635.00 | 172,200.00 | 61 | 119,835.00 | 12 |
| FR92A.00.0 over Hale Brook | 104,700.00 | 130,250.00 | 24 | 113,725.00 | 9 |
| Forest road no. | Road name | Stream name | Drainage area (km2) | Bankfull width (m) based on regional curves | Failed structure type and size | Ratio of structure to bankfull width | Approximate repair cost due to crossing failure ($) | Approximate total road repair cost ($) |
|---|---|---|---|---|---|---|---|---|
| FR394 | Townsend Brook | Townsend Brook | 1.3 | 3.0 | Native stringer bridge with approximately 1.5-m opening | 0.50 | 81,000 | 104,000 |
| FR226 | Corporation Brook | Corporation Brook | 4.4 | 5.0 | 2.0-m culvert | 0.40 | 10,000 | 105,000 |
| FR45 | Chittenden Brook | Chittenden Brook | 14.8 | 8.6 | Bridge 11.6 m | 1.35 | 175,000 | 190,000 |
| FR35 | Upper Michigan | Michigan Brook | 19.9 | 9.8 | 3.7-m culvert | 0.38 | 247,000 | 247,000 |
| FR39 | Texas Falls | Texas Falls Brook | NA | NA | Numerous 46-to 60-cm cross-drain culverts | n/a | 82,000 | 82,000 |
| Totals | 595,000 | 728,000 |
Box 2.5: Testing Adaptation Methods for Forest Management Operations
Climate change is expected to limit operability for carrying out forest management activities. Concerns revolve around longer mud seasons in the spring and fall and warmer winters with less frozen ground and snow cover that may not safely freeze roads and stream crossings, thereby limiting erosion. A joint project between Atlas Timberlands and Vermont Land Trust (Climate Change Response Framework, 2020) tested options to keep operating logging equipment in the midst of climate change by focusing more on preventing problems than fixing them. (Note: This land has since changed hands and the project has ended).
Option 1: Changing equipment. A shift from skidding wood to cut-to-length systems limits impact to the ground and provides incentive to retain slash in the woods. The downside is the expense to change equipment; this change requires a cut-to-length harvester and forwarder.
Option 2: Cut in summer and minimize impacts via improved bridges and other road construction. A greater investment in road construction and portable skidder bridges makes summer logging more feasible (and summer drought could help this), but extra costs are incurred.
Option 3: Pre-manage roads. Construction of smaller water bars (earthen drainage structures that direct water off roads to prevent erosion) before close-out (as opposed to waiting until close-out to install full water bars) and brushing in roads before they get muddy are both ways to protect roads and increase the number of workdays possible at minimal cost.
Outcomes: This case study illustrates that it is cheaper and easier to log during a cold winter (Table 2-5), though, unfortunately, this is becoming less possible. Logging in other seasons is feasible but may take a greater financial investment. The options discussed in this section can increase operability but have limitations, including on private land, where closed logging roads are often used for footpaths, stacked brush on roads and water bars diminish landowner use of the roads and post-harvest satisfaction. Changing equipment requires education, financial investment, and buy-in by loggers that these changes will ultimately benefit their businesses. Options that will help logging contractors adapt include nimbleness (i.e., ability to move between jobs based on site conditions) and purchase of site management equipment (i.e., bulldozers or excavators), both of which come with increased costs. Cost-sharing of equipment and resources like portable bridges may help reduce the cost of these activities.
For forest managers, adaptation options will include a) writing logging contracts to span a longer time to ensure enough time with good operating conditions and b) separating road-building contracts from timber contracts to ensure that road upgrades can be carried out. Determining the best adaptation methods will require cooperation between foresters and logging contractors, as they are on the ground managing site conditions. Adaptation actions by logging contractors will need to be incentivized and supported so that these contractors can adapt their practices to new conditions.
Table 2-5: Estimated costs of shifting from winter to summer harvesting on Atlas Timberlands Operational Adaptation Project
Winter Harvest
| Task | Cost estimate | Notes |
|---|---|---|
| Lower Truck Road | ||
| Fill/elevate – 500′ along wetland | $4,500 | Material from ATP pit |
| Armor ditches | $1,000 | |
| Stone 2 crossover | $1,000 | Shot rock |
| Upper Truck Road | ||
| Cut back brush/trees | $1,500 | |
| Ditching, stumping, widening | $3,000 | |
| Replace culverts (3) | $2,000 | |
| Stone crossover (1) | $ 500 | |
| Misc. | ||
| Skid road construction (main) | $ 500 | |
| Plowing | $. 500 | |
| TOTAL (Winter) | $14,500 |
Summer Harvest
| Task | Cost estimate | Notes |
|---|---|---|
| Lower Truck Road | ||
| Fill/elevate – 500′ along wetland | $ 4,500 | Material from ATP pit |
| Armor ditches | $ 1,000 | |
| Stone 2 crossover | $ 1,000 | Shot rock |
| Upper Truck Road | ||
| Cut back brush/trees | $ 1,500 | |
| Ditching, stumping, widening | $ 3,000 | |
| Replace culverts (3) | $ 2,000 | |
| Stone Crossover (1) | $ 500 | |
| Harvest Area | ||
| Portable skidder bridge | $ 5,500 | Custom: steel, welding |
| Bridge abutments | $ 1,000 | Concrete block |
| Decking | $ 1,500 | Re-use, if possible |
| Installation | $ 1,500 | |
| Job layout time (additional) | $ 2,500 | |
| Misc. | ||
| Skid road construction (main) | $ 5,000 | |
| Pre-harvest waterbars | $ 3,000 | |
| TOTAL (Summer) | $33,00 |
Back to table of contents | See all chapters
2.6 Forest Management Challenges and Opportunities
2.6.1 Ability to Carry Out Silvicultural Operations
Management for wood products, non-timber forest products, wildlife habitat, recreation, and climate mitigation are all dependent on the ability to carry out silviculture, or the planning of goal-based forest management activities around the ecology of the forest and the species that occupy it. Limits to operability in forests may lead to struggles to reach silvicultural goals. For example, sugar maple, a dominant and desired tree in Vermont, regenerates best through leaf litter on the forest floor. Harvesting in snowy conditions leaves the forest floor undisturbed, but as winter harvest becomes more difficult and less common, more ground scarification can occur during harvest, benefitting the regeneration of other species. Beech trees, already impacted by beech bark disease (see disturbance subsection; Cale et al., 2015) will aggressively root sprout with ground disturbance, and forests containing high amounts of diseased beech are often intentionally managed in the winter. The loss of winter logging may have cascading effects on forests, increasing beech sprouting, which will ultimately limit the regeneration of other desired tree species such as sugar maple.
More generally, stressors related to climate change and globalization (e.g., spread of invasives) will require forest management action to aid with ecosystem adaptation. Economic impacts that reduce operator availability, close mills, discourage forest products as a career path, or constrain forest management limit the ability for foresters, who are trained and able to manage forests for adaptation, to carry out management actions that can benefit the forests and rural communities and provide local wood to Vermonters. Wood product consumption tends to remain fairly consistent, so if not sourced locally then wood is being harvested elsewhere, potentially in a location with fewer environmental regulations, greater costs, and increased carbon emissions from longer transportation routes (Berlik et al., 2002).
2.6.2 Active Management vs. Passive Management
As climate change mitigation becomes more of a policy priority, the value of forests as a natural carbon sink is increasingly recognized. This has led to a flurry of recent research seeking to understand how much carbon is stored by forests and which forest types, forest ages, and management approaches are best for climate mitigation. The science of forest carbon is in a state of flux, as new measurements and methods arise to understand carbon dynamics and climate mitigation potential. Passive management, or the decision to not carry out management activities in a forest, has been put forth as the way to maximize forest carbon storage, though not necessarily sequestration (Luyssaert et al., 2008), but recent research indicates that old or passively managed forests may not be storing as much carbon as previously suspected (Gundersen et al., 2021). Additionally, good, active forest management can lead to large healthy trees that, when harvested, convert to carbon storage in durable wood products; this can be a long-term (100-year) solution for keeping carbon out of the atmosphere while still realizing a broad suite of services from the forest (Dugan et al., 2021). Prioritizing the mitigation value of forests by choosing a passive management approach has both positive and negative effects; no one management solution is best for all forests. Funding for carbon credits (see below) can support conservation of forest land, insurance from land use change, and good business practices. High levels of carbon sequestration in forests can offset the carbon emitted by fossil fuel use, but this process can be only one part of climate mitigation and must go hand in hand with reduction of CO2 emissions. Depending on the scale employed, passive management with the sole emphasis of maximizing carbon storage creates the potential to minimize other ecosystem goods and services provided by forests, such as wildlife habitat, wood products, non-timber forest products, water filtration, and recreation.
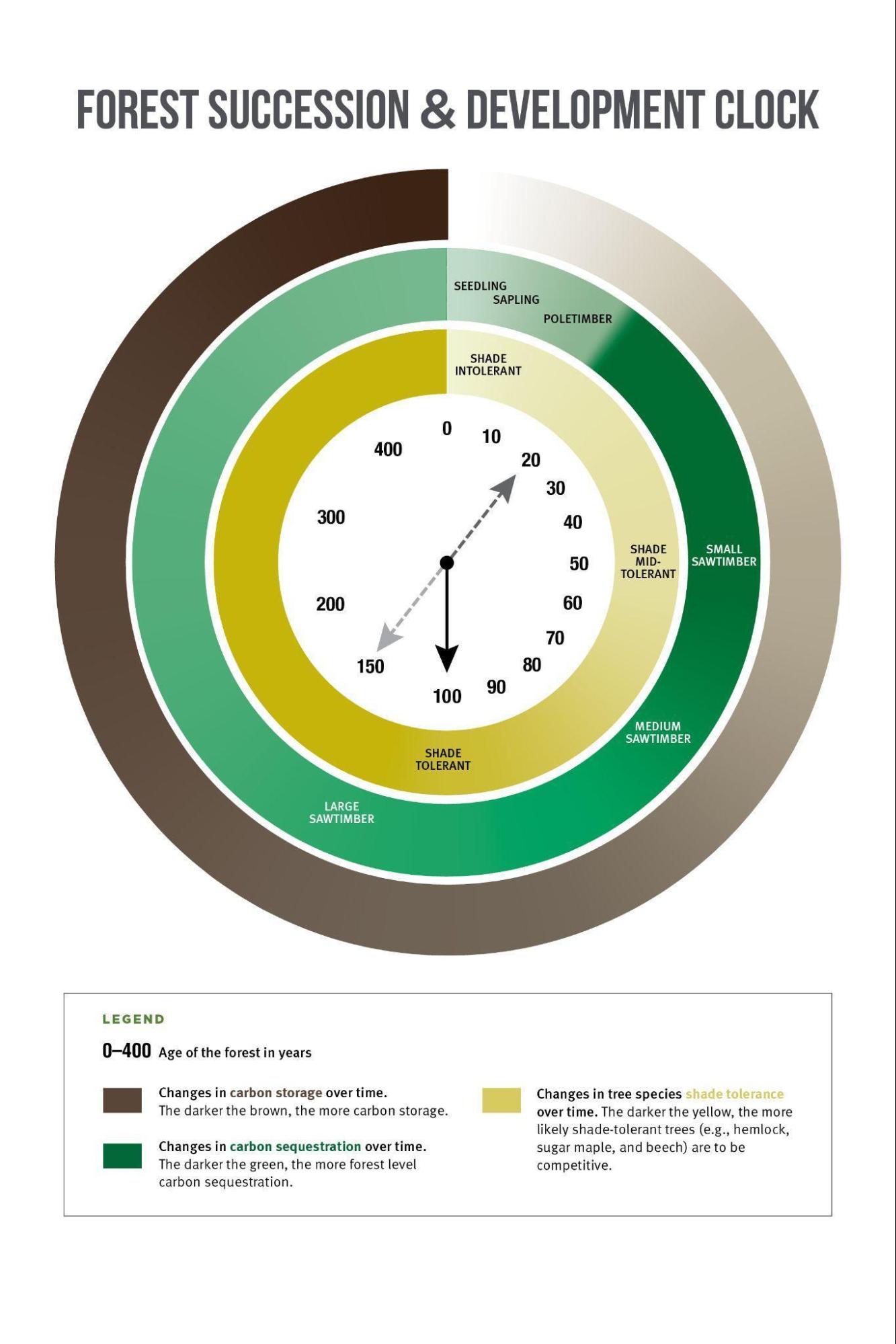
One point of confusion is the differences between carbon sequestration and carbon storage. Sequestration describes the amount of carbon that is actively being removed from the atmosphere, while storage is the amount of carbon that is being held in trees and forest soils. Sequestration and storage have different relationships with stages of forest development: while older forests store more carbon, younger forests tend to sequester more carbon (Figure 2-18). While some research indicates that old forests store a significant volume of carbon, forests are dynamic systems, and old forests do not remain in a steady state. As a result of past land use, Vermont’s forests are predominantly in the eighty to hundred-year age class (see Structure and Composition subsection). This uniformity in age class means that a greater portion of the forests are vulnerable to similar types of disturbances, threatening their ability to store carbon and opening the possibility that vast portions of the forest could revert to a younger state simultaneously, upsetting the current carbon dynamics of Vermont’s forests.
2.6.3 Land Use Change
Written by Jamey Fidel, General Counsel, Forest and Wildlife Program Director, Vermont Natural Resources Council.
Vermont is the fourth most heavily forested state in the country, and while approximately 74% of the state is covered by forests, forests are declining in extent on an annual basis (USDA Forest Service, 2020). While it is extremely hard to pinpoint the exact amount of forest loss, according to the Forest Service, approximately 14,207 acres of forest land are being converted to non-forest every year (USDA Forest Service, 2020) After factoring in land that may revert to forest, at this rate a net of over 300,000 acres of forestland may be converted to non-forest by 2050 (USDA Forest Service, 2019).
As forests become more compromised by development and urbanization, their ability to remain healthy and provide ecosystem services, such as sequestering and storing carbon, will be diminished. For example, a Forest Carbon Assessment documented that the total annual uptake of carbon was less in 2015 (the end of the period of analysis) than in previous decades, in part due to declining acres of forest land (Schultz et al., 2017). A more recent forest carbon inventory confirmed that land use change has resulted in net emissions in Vermont, which is concerning because forest land that is converted not only emits stored carbon, but it also reduces future forest carbon sequestration (Kosiba, 2021a).
As large undeveloped forests are broken into smaller and smaller parcels from subdivision, forest management becomes more challenging, and the relationships between parcel size and forest owner behaviors have important implications for timber supply, resiliency, restoration, and keeping forests as forests (Butler et al., 2021). Data gleaned from the Grand List in Vermont highlights that undeveloped woodland as a land category decreased significantly from 2004 to 2016, while residential acreage increased by almost 162,670 acres (Fidel et al., 2018). During the same period, the amount of land in parcels 50 acres or larger declined by about 110,300 acres, while the number of parcels under 50 acres in size with new houses increased by 20,747 parcels (Fidel et al., 2018). This highlights an increasing trend in Vermont: undeveloped forest land is being converted to residential development with houses and associated infrastructure, and smaller parcels are being created through the fragmentation and parcelization of forestland from subdivision and development. Left unaddressed, these trends will limit the ability of forests to remain resilient and provide vital services to mitigate the impacts of climate change.
2.6.4 Carbon Markets
Vermont’s Department of Forests, Parks, and Recreation has created comprehensive reports explaining forest carbon, the carbon market, and its status and relevance in Vermont (Kosiba, 2021b). Here, carbon offsets as a forest management opportunity for Vermont’s landowners are summarized.
Forest carbon offsets aim to reduce global CO2 emissions by taking advantage of forests’ ability to sequester and store carbon. Carbon offsets put a market value on sequestration or storage to make up for emissions of CO2 in cases where CO2 reduction is impossible or undesirable. Carbon offsets allow companies or individuals to offset their own carbon emissions by paying to guarantee that carbon elsewhere is sequestered (actively removed from the atmosphere and stored in another form or location) or stored (remaining in another form or location, preventing its release back to the atmosphere). The key to effective carbon offsets is that the payment is responsible for carbon sequestration or storage that would not have happened or continued without it. Because forests are excellent at storing and sequestering carbon, they are an opportunity for CO2 emitters to offset their climate impacts. And because forests cover much of Vermont, the growing market for carbon offsets is an economic opportunity for Vermont’s forest landowners.
Carbon offsets, also known as carbon credits, are treated as a currency in units of the equivalent of 1 metric ton of CO2. A metric ton is about the amount of CO2 emitted by an American car driven regularly for two-and-a-half months or the amount of carbon sequestered by forty-six mature trees in a year (US EPA, 2015). Carbon offsets are bought and sold on carbon markets or registries, of which there are several, each with somewhat different restrictions and requirements. Some carbon markets are regulatory, used as a tool to help large emitters in states like California meet state CO2 emission guidelines, while others are voluntary, where companies and individuals choose to pay to offset their emissions. Over the past several decades, an industry has grown around these carbon markets, so there are now organizations and consultants—such as carbon developers and third-party verifiers—engaging in every step of the process that facilitate these transactions.
Forest carbon offset projects fall into one of three categories: 1) afforestation or reforestation (tree planting), 2) avoided conversion (blocking the clearing of already-forested land), and 3) improved forest management (shifting management practices to allow an existing forest to store and/or sequester more carbon than before). Improved forest management is the most relevant to Vermont’s forest landowners. Through a variety of management techniques, both active and passive, forests are intentionally managed to maintain their carbon storage at or above an agreed-upon level.
Two key components of carbon offset projects are permanence and additionality. Permanence means carbon must be continually stored for the duration of the project’s contract, often from 40 to 100 years, and the carbon stored must remain above and beyond the amount that is stored under a “business as usual” scenario, which is typically benchmarked at a baseline year. Additionality is the difference between the increased carbon stored in the offset-insured forest and the baseline of carbon stored without the offset incentive. Determining baselines for forest carbon offset projects can be complicated, and regulations vary among carbon markets. Defining the baseline for a carbon offset project depends on a specific property’s circumstances, including legal encumbrances (i.e., easements or deed restrictions) and harvesting regulations. For example, a forest previously protected by a “Forever Wild” conservation easement with no logging allowed may not qualify for a carbon offset project; as the forest is already guaranteed to remain unharvested, there is no additionality possible through an improved forest management project.
However, forest carbon offset projects are compatible with existing programs in other ways. Protecting a forest with a conservation easement at the same time it enters the carbon market can help ensure the permanence of carbon-centric management of the parcel. Forests enrolled in Vermont’s popular Use Value Appraisal (or Current Use) program, which provides a tax benefit for actively managed forestland, may also enter a carbon offset project, provided the requirements of both programs continue to be met.
Since carbon markets are varied and evolving, contacting a carbon developer is the best way for interested landowners to learn about the process and whether it may be right for them. The most established carbon registries typically deal with large forest parcels (>2,000 acres), but new and emerging companies are extending this opportunity to smaller forest landowners. Under the right circumstances, projects that aggregate many small landholdings may be appropriate to join the carbon market (see Box 2.6). Small landowners may also benefit from emerging practice-based carbon programs such as the Family Forest Carbon Program being developed by the American Forest Foundation and The Nature Conservancy.
Box 2.6: Cold Hollow to Canada
Forests provide a multitude of ecosystem benefits including wildlife habitat, improved water and air quality, and recreational and aesthetic benefits to humans. Fragmentation diminishes these benefits. For example, the habitat of some birds that nest in interior forests is greatly reduced when these forests are broken up into smaller patches with more exposed edges and less interior. Therefore, small, privately owned forest parcels pose a challenge to conservation; if one of those parcels is developed, the impacts extend to the surrounding forest as well (Baldwin and Fouch, 2018). Landowner cooperation has other benefits, too: sharing resources, equipment, and services such as management planning contribute to more cohesive management and lower costs for individual forest owners (Kittredge, 2005). Carbon markets are set up to include large forest parcels; programs that work with smaller areas are few and still emerging. At this time, managing small, adjacent forest parcels as an aggregate is beneficial both to the humans and animals who use these forests and to the individual landowners who have more options to enter the carbon market.
Beginning in 2018, the Vermont Land Trust and Cold Hollow to Canada coordinated the aggregation and sale of carbon offsets for the forests of ten private landowners. This cooperative project is the first of its kind in the United States. Individual landowners with properties too small to be lucrative on the existing carbon market worked together with partner organizations to sell carbon offsets as a group for their forested land totaling over 7,500 acres (Hancock, 2020). Although historically, only large forest parcels of thousands of acres were economically viable in the carbon market, the Cold Hollow to Canada project represents a way forward for smaller landowners in Vermont and beyond. By banding together, the connected forests are protected as a unit for at least forty years. And by managing for carbon storage, many other ecosystem benefits result; for example, improved water quality and wildlife habitat are amplified by the larger footprint of this project’s carbon-rich forests.
Back to table of contents | See all chapters
2.7 Forest Adaptation and Management
Forest adaptation describes the capacity to respond to new or novel conditions. Uncertainty is a recurring theme when it comes to forest adaptation to climate change. Over very long historic time scales (thousands of years or more) forests responded to changing climate conditions and persisted, but climate changes now are happening on the scale of decades, so there is concern that forests may not be able to adapt at that pace. In response, forestry has adopted adaptive management, which includes changing forest management approaches in response to changing local conditions and is driven by research and practitioner action.
Forest adaptation and management can be understood within a matrix of change and response, with approaches laid out along a continuum (Figure 2-19; Millar et al., 2007). In forest adaptation and management, there is not one simple solution; rather, site-specific decisions are made based on local conditions and dynamics, management objectives, and best available knowledge about future change. Adaptation requires embracing uncertainty and figuring out the best options out of those available. In the context of forest adaptation and management, a resistant forest remains consistent in structure and function through change, whereas a resilient forest accommodates some change but retains the capacity to return to a desired reference condition. A transition forest is in the process of adapting to new conditions and eventually may look quite different from what is currently on site, though it still performs similar ecosystem functions.
Adaptive capacity of forests is based on forests’ structure, composition, and function; adaptive management approaches target these components of forest ecosystems (Table 2-6). In many cases adaptive management can be carried out in concert with ecological forestry practices, focused on mimicking natural disturbances and processes (D’Amato and Palik, 2020). Forest adaptation is a recent research focus; while it builds on basic ecological concepts, its application is in its early years. Detailed adaptive management approaches are available online from the USDA Forest Service Climate Hubs as an adaptation workbook (NIACS, 2021).
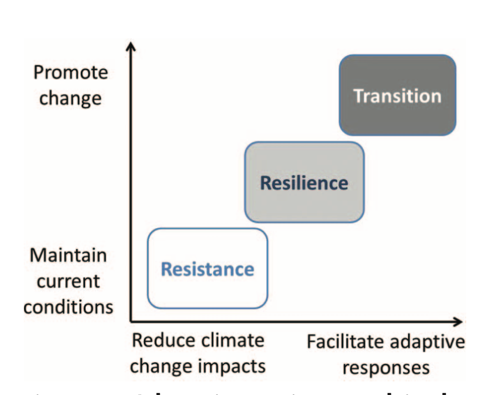
Table 2-6: Examples of adaptive management techniques
| Forest Adaptive Management for Climate Change Adaptation | |||
|---|---|---|---|
| Tactic | Example | Pros | Cons |
| Structural retention | Retain large old trees, both living and dead, during timber harvest | “Lifeboat” for species diversity, continuity of forest structures and processes, carbon storage in larger trees, habitat for cavity nesting birds and invertebrates that feed on deadwood | Potential loss of revenue, retaining large trees can create unsafe conditions for operators |
| Extended rotations | Regenerate forest after 100 years instead of 80 years | Carbon storage, multiple age classes | Potential revenue loss, older forests more vulnerable to disturbance |
| Increase structural complexity | Intentional creation of horizontal and vertical heterogeneity, tree species, size classes, deadwood decay classes, spatial arrangements | Greater habitat diversity, variety of disturbance recovery pathways | Costs may be limiting, complicated operational layout |
| Increase functional redundancy and diversity | Include large canopy gaps, retention, and uncut patches to increase diversity of regeneration niches at a stand scale, retain uncommon species | Persistence of rare functional traits through novel disturbance | Complicated operational layout |
| Increase habitat connectivity | Treat large parcels in a nonuniform manner, plan forest management activities at a landscape scale when possible | Multiple habitat types near each other and connected | Requires cooperation from multiple adjacent landowners |
| Assisted migration | Plant species from southern New England in gaps created by timber harvest | Forest populated with future climate-adapted species | Efficacy and safety not yet supported by long-term data |
Box 2.7: Early Lessons from Adaptation Plantings Aimed at Transitioning Species Composition for Climate Change in the Northeastern United States
Written by Peter Clark, University of Vermont
In 2018, adaptation plantings were installed in harvest gaps in northern hardwood and mixed conifer-hardwood forest across sites in Vermont and New Hampshire as part of the New England installation of the Adaptive Silviculture for Climate Change project (adaptivesilviculture.org). The goal of this experiment was to examine the performance of future-climate adapted tree species from a suite of functional traits (e.g., seed mass, shade tolerance, growth rates) to better understand the relationship between introduced species and contemporary drivers that may control seedling growth and survival.
The future-adapted species (“adaptation plantings”) tested included a) “population enrichment” plantings (northern red oak, black cherry, red spruce, white pine, eastern hemlock, and bigtooth aspen), which were generally underrepresented onsite but have ranges that encompassed research sites (Figure 2-20) and b) “assisted range expansion” plantings (black birch (Betula lenta), bitternut hickory (Carya cordiformis), and American chestnut (Castanea dentata), which were currently not onsite but planted with modest advances outside of current range but within future projected habitat range.
Over three growing seasons, 3,152 out of 5,620 total seedling transplants survived (approximately 57%). Seedling growth and survival varied considerably among species, with slight inverse relationships (i.e., some species grew faster, while others had higher survival rates; Figure 2-21). The major factors influencing seedling performance were competition from shrubs and herbaceous plants, species regeneration traits, initial size and health of seedling, and transfer distance from species range.
Sites with strong “ecological memory” in the form of dense natural regeneration were more likely to outcompete the new or novel adaptation plantings; however, sites with heavy deer browse slowed down natural competition, which inadvertently favored many adaption plantings. Moreover, species traits moderated seedling response, such as the ability to root-sprout after winter injury or dieback (e.g., hickory and chestnut), rapid initial growth to outcompete vegetative competition (e.g., aspen, cherry, and pine), energy allocation to roots (e.g., red oak), and inherent deep shade tolerance to persist under competition (e.g., spruce and hemlock).
Population enrichment species performed significantly better than assisted range expansion species, which exhibited more maladaptation, further highlighting the challenges of assisted migration. Additionally, these adaptation plantings experienced extreme climate events, such as a once-in-a-century spring drought during planting and late spring frosts in subsequent years that caused negative consequences on seedling performance that differed by species and across regional sites. Extreme climate events like these are important future climate analogues for conditions that managers will contend with as they consider implementation of adaptation plantings.
Forests in the Northeast are projected to experience profound changes in suitable tree habitats, highlighting the potential importance of adaptation plantings. Assisted migration activities may be necessary to establish future-adapted trees, so the strength of local site competition, adaptive traits, assisted migration distance, and availability of quality and diverse nursery seedling stock will play important roles in determining performance and efficacy of efforts to transition forest composition.
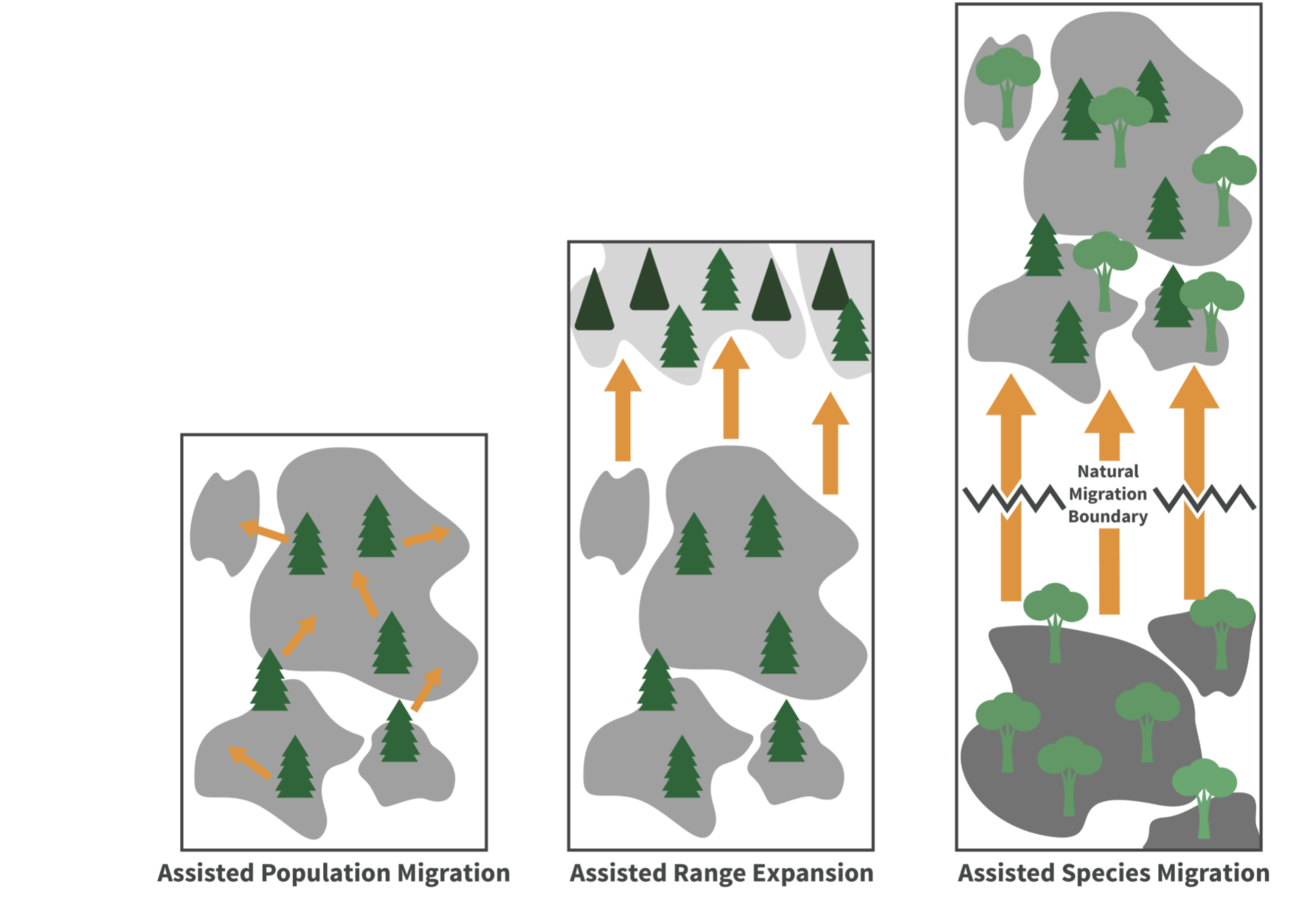
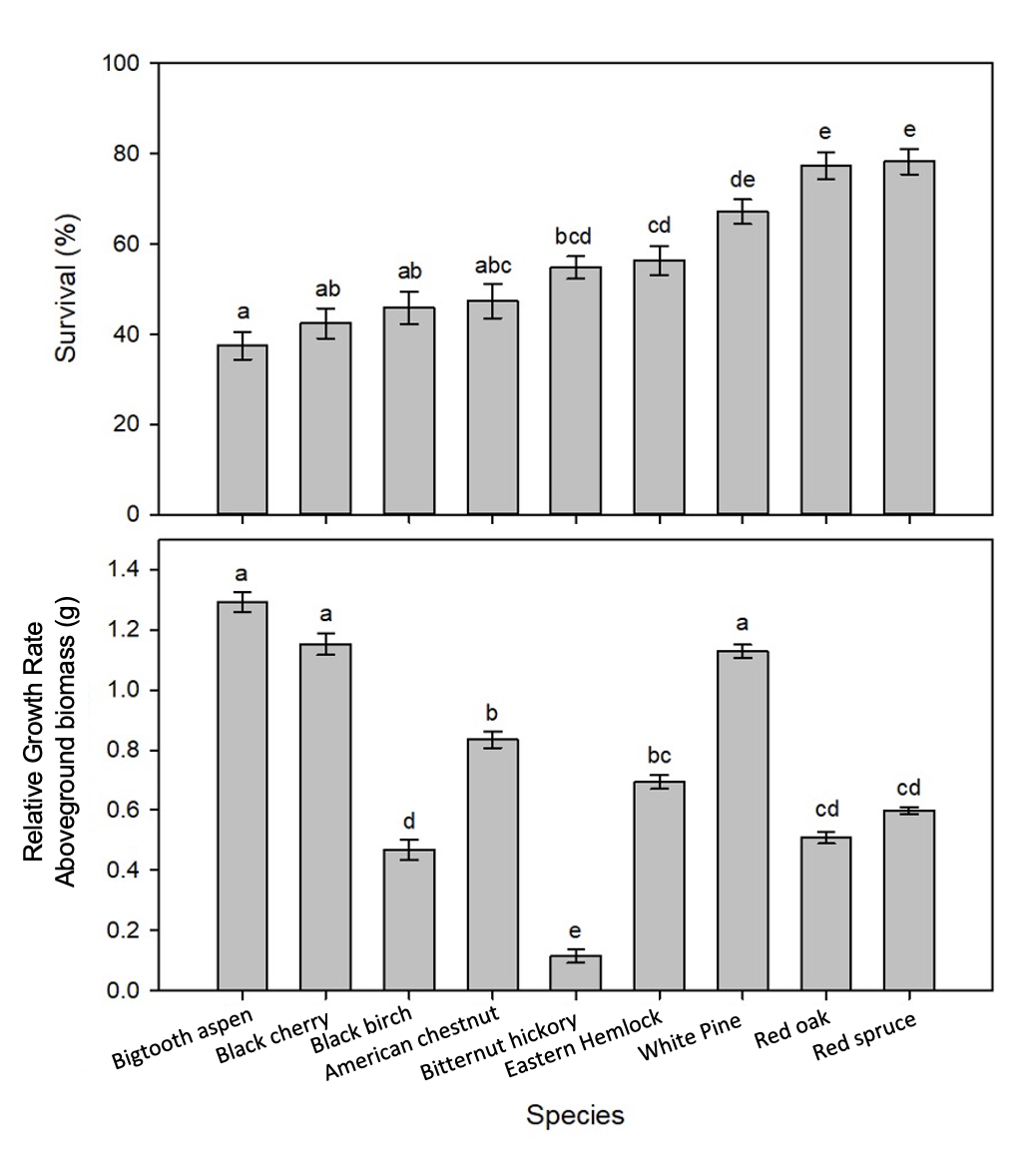
Note: Growth is measured as the relative annual growth rate in above ground biomass (grams). Letters denote significant differences (α ≤ 0.05)
Box 2.8 Adaptive Silviculture in Response to Emerald Ash Borer and Climate Change
The emerald ash borer (EAB, see Disturbance subsection) will have significant impacts on Vermont forests with ash populations. Forest management may either exacerbate or mitigate impacts. In conjunction with Dartmouth College, University of Vermont researchers are examining adaptive silviculture strategies with the goal to help forests with a significant ash component maintain ecosystem function in the face of this invasive pest and climate change. The research site in Corinth, Vermont is a rich northern hardwood forest dominated by sugar maple with a significant white ash component (Figure 2-22). Silviculture treatments were co-produced with input from multiple interested parties, including the Cowasuk band of Indigenous Abenaki. The silviculture treatments are designed to support a structurally and compositionally complex forest characterized by multiple combinations of species composition and structure. A forest with this structure will have multiple pathways to recover from disturbance, including disturbance created by EAB. In practice, this means creation of five age classes through time, achieved by group selection treatments creating gaps from one-tenth to one-fourth of an acre in size; intentional creation of downed dead wood and cavity trees, achieved by retaining slash on site and leaving uncut large ash trees that are likely to die; planting future climate-adapted species not currently existing on site (e.g., basswood, northern red oak, bitternut hickory, black cherry, bigtooth aspen); and releasing crop trees of basswood, healthy female ash trees, and vigorous, resistant specimens of other species. The treatments will also serve to increase songbird habitat by increasing diversity in vertical and horizontal forest structure, to maintain forest productivity, and to diversify microhabitat conditions to enhance abundance of understory vascular plants, including those of cultural significance to the Cowasuk band of the Abenaki.

Back to table of contents | See all chapters
2.8 Urban Forests and Climate
There are an estimated 11.9 million trees in Vermont’s urban and developed areas (Nowak and Greenfield, 2008). While these areas make up less than 2% of the state’s land area, nearly 39% of the Vermont population (243,000 people) lives in a census-defined urbanized area (U.S. Census Bureau, 2012). All trees—whether growing along a street or in a forest—provide critical climate and ecosystem benefits, like carbon sequestration, water infiltration, temperature moderation, erosion control, and pollution abatement. Because of the high population density and lower tree cover in urbanized areas, per-tree ecosystem services in urbanized areas can be higher than in forest settings. Yet because of the amplifying effects of the built environment on temperature and water cycling, along with additional stressors associated with urbanized areas like soil compaction, poor soil fertility, and pollution, urban trees are highly vulnerable to climate change.
2.8.1 Urban Forests and Carbon
Like forest trees, trees growing along roads and in yards, parks, and community forests provide important climate mitigation effects by sequestering and storing atmospheric CO2 in wood and soil. Unfortunately, there is no standard definition of what constitutes an urban or community forest, so it is difficult to compare mitigation estimates from various sources. Additionally, estimating tree carbon, especially annual sequestration, is challenging and imprecise. It requires modeling a tree’s biomass based on the carbon in a reference set of sample trees. These models can be inaccurate for street and yard trees because the growing conditions are highly variable compared to forest-grown reference trees (McHale et al., 2009; McPherson et al., 2016). Finally, to compute the actual climate mitigation effect of a tree, the maintenance inputs should be included (Nowak et al., 2013), and this is often either unknown or highly variable.
Despite these challenges, several sources have estimated the total carbon storage and annual sequestration of Vermont’s urban trees. According to these estimates, trees in urbanized areas store about 15 MMt CO2e (equivalent to the annual emissions from 3.8 coal fired power plants) and sequester 157,000–500,000 Mt CO2e per year (equivalent to 18,000-60,000 U.S. homes’ annual energy use) (Domke et al., 2020; EPA, 2021; Nowak et al., 2013; Zheng et al., 2013). On a per area basis, some estimates suggest that Vermont’s street trees sequester CO2 at a higher annual rate compared to forest trees (Nowak et al., 2013), as urban trees tend to have wider tree crowns, experience less competition from other trees, and have a greater leaf area compared to forest-grown trees. However, urban trees have significantly shorter lifespans and greater maintenance demands, which affect their lifetime carbon storage potential and resultant mitigation benefit (Nowak et al., 2013). After accounting for the greenhouse gas emissions from growing, planting, and maintaining urban trees, one study found that an urban tree must live a minimum of ten years to provide a net positive mitigation benefit (Nowak et al., 2013).
In addition to the direct benefit of sequestering atmospheric CO2, trees in urbanized areas provide important indirect climate benefits. All trees moderate temperature fluctuations by shading surfaces and transpiring water vapor, but in urban areas this is particularly important because of the urban heat island effect (Millward et al., 2014; US EPA, 2014). A tree’s canopy provides shade and wind protection for buildings, which reduces energy needs. This results in lower greenhouse gas emissions and reduced heating and cooling costs (McPherson and Simpson, 1999; US EPA, 2014). Trees also act as green infrastructure, reducing stormwater runoff from extreme rainfall events by transpiring water and keeping soil intact with their roots (Nowak et al., 2020).
2.8.2 Specific Climate Change Impacts on Urban Forests
Trees in urbanized areas experience different and often intensified impacts from climate change because of the built environment. Urban areas may experience hotter and drier climates than interior forests (Fahey et al., 2013). There are more impervious surfaces that cause stormwater runoff (Solecki and Marcotullio, 2013). Under a warmer climate, we may see more ice and windstorms, which can damage trees, infrastructure, and property (Dale et al., 2001; Neumann et al., 2015). Urban trees may be weakened by compounding stresses of heat, drought, extreme weather events, and pests and pathogens, which may not only decrease vigor and growth, but increase mortality. This, in turn, reduces the ability of urban trees to sequester and store carbon (Figure 2-23). A study from Cambridge, Massachusetts found that climate-related changes could result in 58% tree mortality (Foran et al., 2015). Current and future management responses will influence the vulnerability of urban trees and forests.
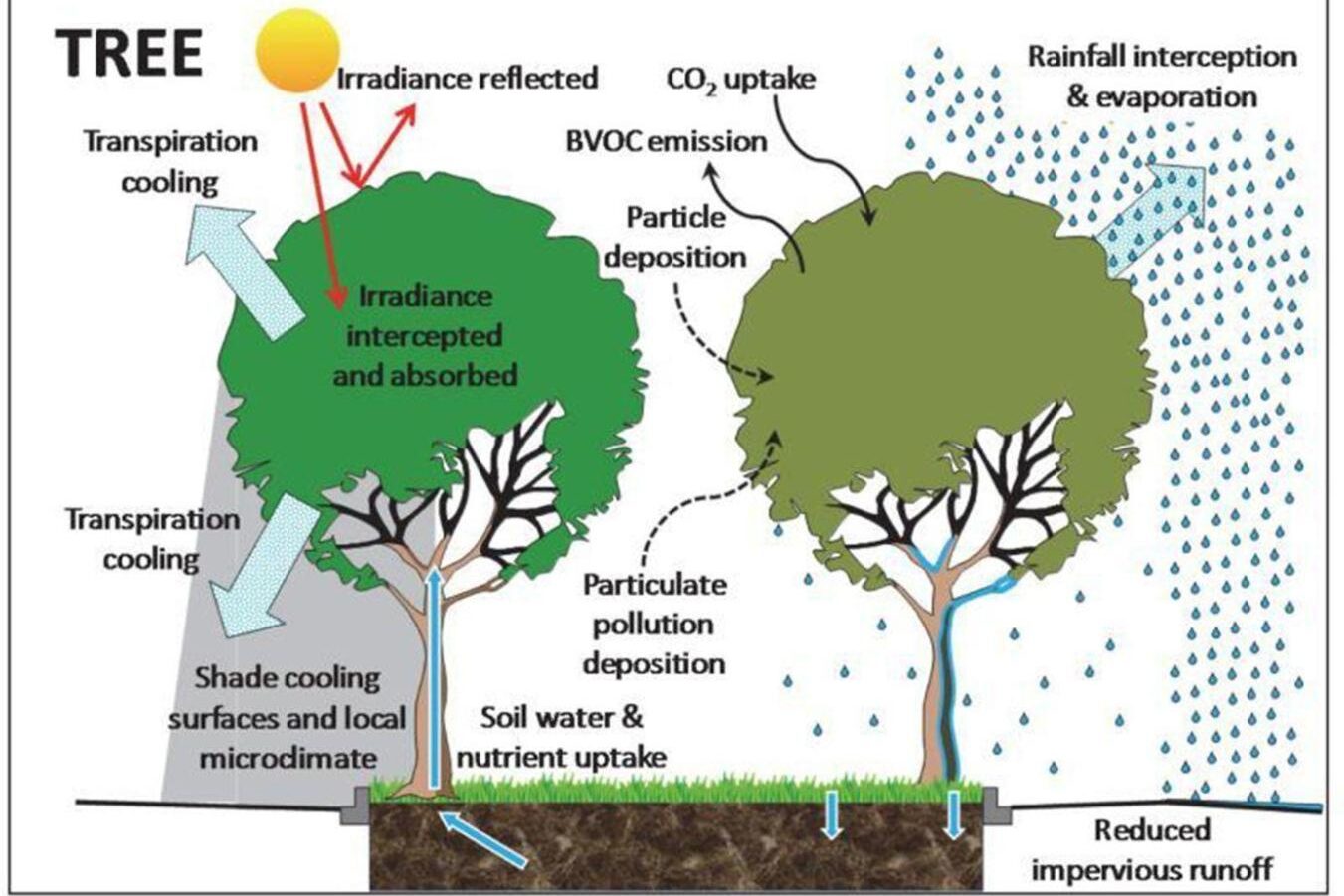
Invasive pests, pathogens, and plants also pose threats to Vermont’s urban trees and community forests. Invasive insect infestations—often targeting specific tree species—strengthen the case for planting a diverse and resilient urban forest as they may eliminate entire swaths of urban-tolerant trees. Climate-related stressors on urban trees—such as drought—may increase an individual tree’s susceptibility to infestation or disease (Tubby and Webber, 2010) and warmer winter temperatures create conditions for some tree pests and diseases to thrive. Competition from invasive plants—less of a concern for street tree populations but certainly relevant to forested parks—impedes natural regeneration of native forest species and has reverberating impacts on natural communities, wildlife, and the local economies that depend on these wooded landscapes for tourism and recreation (Milanović et al., 2020).
There are numerous examples of the impacts of invasive insects and disease on urban trees. Many of Vermont’s downtowns experienced widespread loss of American elm (Ulmus americana) in the mid-twentieth century due to Dutch elm disease (Ophiostoma spp.). Now many of these same communities are preparing for the loss of ash trees—particularly planted green ash (Fraxinus pennsylvanica)—from streets and parks due to the invasive emerald ash borer, which was first detected in Vermont in 2018. According to the Vermont Urban & Community Forestry Program’s (VT UCF) inventory of public urban trees (>24,000 trees across thirty municipalities), approximately one out of every six Vermont downtown and neighborhood trees is an ash. Additionally, municipal staff and volunteers in more than forty towns and cities have recorded over 45,000 ash trees along rural roadsides using VT UCF’s Rural Roadside Ash Inventory Tool (VT UCF, 2021a).
Together, these stressors add complexity to the already daunting task of selecting and sourcing urban-tolerant tree species for planting and of moving the needle towards diverse species composition in urban areas. Only four Vermont municipalities have an arborist on staff. This means that in most communities, tree planting and maintenance efforts are led by citizen volunteers or are contracted to outside organizations. Vermont’s limited number of tree nurseries are challenged with selecting and propagating species that will be successful in an uncertain future. Until recently, there has been little attention to genotypes (genetic adaptation) when breeding trees for planting. Even within a species there is significant variability in physiological response to climate and site factors, such that seed source is an important consideration under a changing climate. Resources to aid these decision-makers are increasingly important to steward long-lived tree species that can provide sustained benefits for urban populations. The US Forest Service’s Climate Change Tree Atlas is an example of one such resource that supports strategic selection of tree species that have high adaptive capacity in the face of climate change (Peters et al., 2020).
2.8.3 Adapting Urban Forests
Worldwide, many cities, regions, and even countries are assessing existing canopy cover using advanced spatial analysis tools (e.g., aerial imagery, satellite imagery, LiDAR) and establishing ambitious urban tree canopy (UTC) cover goals as part of climate action plans. In Vermont, the University of Vermont’s Spatial Analysis Lab continues to build its database of remote imagery and pioneers UTC work at a national level. The VT UCF program has funded UTC assessments in Montpelier, St. Albans City, Rutland City, and South Burlington, and two UTC assessments for the City of Burlington to assess change over time (VT UCF, 2021b). In concert with UTC goals is the recent proliferation of million and even trillion tree-planting initiatives. This collective recognition of the power and importance of urban forestry is encouraging, but they are only worth engaging in if they are done well. Investment in the proper care of urban and community forest trees is vital. Large, long-lived species that have been maintained for structural integrity will provide the most benefits over time (Nowak et al., 2002).
As Vermont urban and community forestry managers consider the role that trees will play in strengthening communities’ resilience to climate change, they must also plan to adapt to climate change. Fortunately, there are resources—many online and free—to support climate change resilience and adaptation efforts. The US Forest Service’s Vibrant Cities Lab and Climate Change Resource Center and the Northern Institute of Applied Climate Science’s Urban Forestry Climate Change Response Framework support climate change-informed urban forestry planning (NIACS, 2021; USDA Forest Service, 2021) The i-Tree suite of tools quantifies the values provided by trees and aids in planning at multiple scales (i-Tree, 2021) New resources like American Forests’ Tree Equity Score Tool and the US Forest’s Urban Forest Inventory and Analysis (FIA) program support data-driven strategic tree-planting plans so that all people have access to the vast benefits that tree canopy provides (American Forests, 2021; USDA Forest Service, 2021). Organizations such as City Forest Credits are even engaging in efforts to legitimately fund urban forestry through carbon offsets.
While urban and developed areas cover smaller areas of Vermont than other states in New England, future expansion of developed areas is likely. These expansions could result in conversion of forests and other natural and working lands to developed lands, reducing tree cover and forest regeneration while increasing impervious surfaces. Land use planning with a climate adaptation lens that includes healthy trees and forests will be critical to ensuring resilience to climate change.
Box 2.9: Spotlight on Vermont Urban and Community Forestry Program
The Vermont Urban & Community Forestry Program (VT UCF) was established in 1991 as a collaborative effort between the Vermont Department of Forests, Parks, and Recreation and University of Vermont Extension. The program provides technical, financial, and educational assistance to roughly 100 municipalities each year to support the management and stewardship of these forest resources (Figure 2-24).
Since 2017, the program has given 1,250 free containerized trees to 645 households in seven priority municipalities through a partnership with the Arbor Day Foundation’s Community Canopy Program (formerly known as Energy-Saving Trees). The Vermont Department of Health’s Climate and Health Program has contributed as a funding partner for multiple years, forging a cross-agency collaboration that recognizes the links between tree canopy cover, public health, and the climate-related services trees provide.
Research from the Climate and Health Program has informed partner selection for VT UCF’s Vermont Community Canopy Program with the goal to provide free trees to communities that are most vulnerable to heat-related illness. The Vermont Heat Vulnerability Index draws together seventeen different measures of vulnerability in six different themes: population, socioeconomic, health, environmental, climate, and heat illness. The municipalities engaged in the program in its first four years are Barre City, Bennington, Bradford, Brattleboro, Newport City, Rutland City, and St. Albans City.
Residents select up to two free trees and use the Arbor Day Foundation’s software interface to identify the ideal tree planting locations on their property to maximize the air, water, energy, and carbon benefits of their tree(s). VT UCF coordinates community tree pick-up events and provides guidance on proper tree planting and care to the participants. The Arbor Day Foundation provides an impact report (see graphic below) to communicate quantified benefits of the program over time. VT UCF intends to continue to offer this program to targeted municipalities annually.
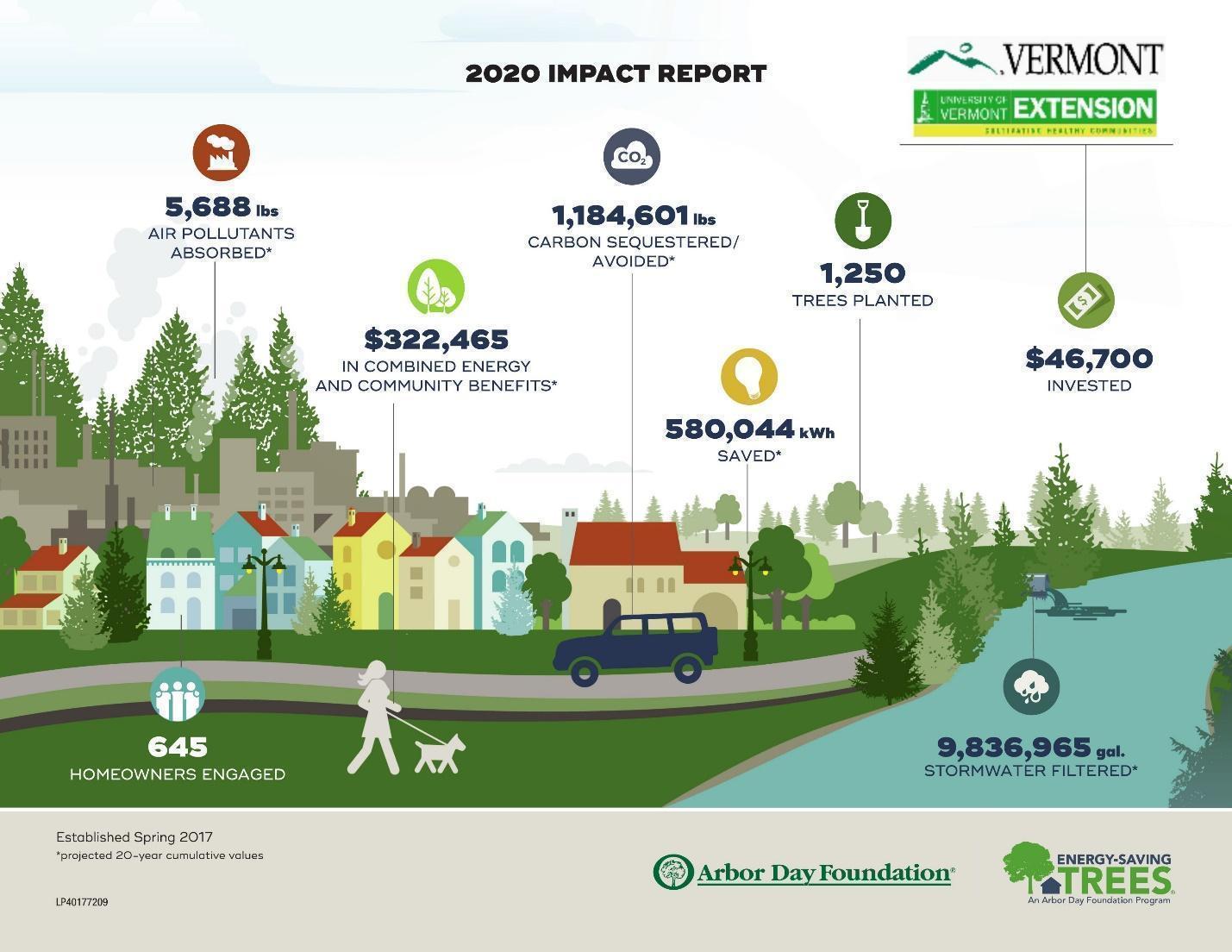
Back to table of contents | See all chapters
2.9 Traceable Accounts
Traceable accounts describe the confidence level—the degree of certainty in the scientific evidence—for each key message resulting from this chapter. This analysis is based on the U.S. Global Change Research Program guidance in the Fourth National Climate Assessment (USGCRP, 2018).
Confidence Level
- Very High – Strong evidence (established theory, multiple sources, confident results, well-documented and accepted methods, etc.), high consensus
- High – Moderate evidence (several courses, some consistency, methods vary, and/or documentation limited, etc.), medium consensus
- Medium – Suggestive evidence (a few sources, limited consistency, models incomplete, methods emerging, etc.), competing schools of thought
- Low – Inconclusive evidence (limited sources, extrapolations, inconsistent findings, poor documentation and/or methods not tested, etc.), disagreement or lack of opinions among experts
Key Message 1
Climate change is expected to shift growing conditions for forests in Vermont, becoming more favorable for southern-adapted tree species and less favorable for cold-adapted tree species. Species that will benefit from this change include northern red oak, shagbark hickory, and black cherry. Species including sugar maple, balsam fir, yellow birch, and black ash will be negatively impacted. While growing conditions will be significantly different by 2100, actual change in forest makeup will follow, as older trees die and are replaced by young ones.
- Confidence level: High
- Major uncertainties: Modeling the future is inherently uncertain. While models agree on the general trend that Vermont’s forests will become increasingly suitable for southern-adapted tree species, the particulars of how this shift will play out are uncertain.
- References: Iverson et al., 2008, 2019; Nevins et al., 2021; Peters et al., 2020
Key Message 2
Forest productivity, or the accrual of plant biomass, is expected to increase in Vermont in the short term, but productivity will be highly variable between species and will likely decrease overall. Factors impacting productivity positively include longer growing season length and CO2 fertilization. Factors impacting productivity negatively include high summer temperatures, short-term drought, nutrient loss from soils, and atmospheric deposition. Productivity is an important indicator of forest health and carbon sequestration and storage.
- Confidence level: Medium
- Major uncertainties: Modeling suggests that productivity will increase under climate change, but modeling is inherently uncertain. It is more likely that the factors decreasing productivity will increase with climate change and outweigh the benefits provided by the lengthened growing season and elevated CO2 levels.
- References: Campbell et al., 2009; Contosta et al., 2017; Duveneck et al., 2016; Norby et al., 2010; Ollinger et al., 2008; Pardo et al., 2011
Key Message 3
Climate change is expected to continue worsening threats from invasive plants, insects, and diseases to the health of Vermont’s forests. These threats are compounded by other climate-related factors, such as worsening storms and increasingly irregular precipitation.
- Confidence level: High
- Major uncertainties: Newly introduced forest pests or pathogens are likely, and their interactions with climate are unknown. Severity of biotic disturbances rely on multiple factors including temperature, precipitation, land use/human intervention, and the specific interactions with each species.
- References: Dobson and Blossey, 2015; Lovett et al., 2016; McAvoy et al., 2017; Seidl et al., 2014; Slippers et al., 2011; Stephanson and Coe, 2017
Key Message 4
Warmer winters and wetter summers brought on by climate change are already limiting active forest management by shortening the time frames that forest operations can take place on the ground. Ground conditions for forest management are projected to worsen, potentially leading to cascading negative effects on rural economies, forest product markets, and management for forest health and climate adaptation.
- Confidence level: High
- Major uncertainties: Locally specific climate effects will be quite variable, so it is hard to project site-level forest management effects. Rural economies and forest products markets may show resiliency in their responses to diminished forest product availability.
- References: Bick et al., 2019; Contosta et al., 2019
Key Message 5
Land use change and parcelization, specifically conversion to residential or commercial use, are a major threat to forest health and productivity, release stored carbon, and potentially limit both ecosystem function and ability of forests to mitigate climate change through carbon uptake.
- Confidence level: Very high
- Major uncertainties: Predicting future human migration patterns has inherent uncertainty. The science of carbon storage is continually evolving.
- References: Fidel et al., 2018; Schultz et al., 2017; USDA Forest Service, 2020
Key Message 6
Increasing forest adaptive capacity through forest management can help forests retain ecosystem function during a changing climate. Although forest adaptation is a new and evolving field, current methods to achieve increased adaptive capacity include increasing forest structural complexity and enhancing compositional and functional diversity and redundancy.
- Confidence level: High
- Major uncertainties: Forest adaptive capacity and adaptive silviculture can increase forest resilience, but research outputs indicating specific impacts are limited, as this is a relatively new field.
- References: Messier et al., 2019; Millar et al., 2007; Nagel et al., 2017
Key Message 7
Climate change impacts will be more severe for urban trees. Urban trees are highly vulnerable to climate change because of the effects of the built environment on temperature and water cycling, and additional stressors associated with urbanized areas like soil compaction, soil fertility, and pollution.
- Confidence level: High
- Major uncertainties: None
- References: Fahey et al., 2013; Foran et al., 2015; Solecki and Marcotullio, 2013; Tubby and Webber, 2010
Key Message 8
The importance of Vermont’s urban trees under a changing climate will be increasingly important to humans because of the services they provide. Because of the high population density and lower tree cover in urbanized areas, per-tree ecosystem services can be higher than in a forest setting. In addition to critical climate and ecosystem benefits, urban trees mitigate the urban heat island effect through cooling and shading and reduce stormwater runoff along impervious surfaces from extreme rainfall events.
- Confidence level: High
- Major uncertainties: None
- References: Millward et al., 2014; Nowak et al., 2020, 2013; US EPA, 2014
Back to table of contents | See all chapters
2.10 Acknowledgements
We would like to thank the reviewers who took time to provide feedback on earlier drafts of this chapter: Tony D’Amato, Jamey Fidel, Jared Nunery, Ali Kosiba, and Jim Shallow. In addition, we are grateful to the many stakeholders who shared their thoughts on the needs for information about how climate change impacts Vermont’s forests and vice versa. We would also like to thank the Northeast Climate Adaptation Science Center and the D’Amato lab for support provided to authors Wikle and Higgins throughout this writing process.
2.11 Resources
As a subset of the references listed below, the following resources are likely to be useful for readers interested in practical information and more detail about the topics discussed.
- Climate Change Tree Atlas provides further information about predicted suitable habitat shifts and adaptability for individual tree species.
- VTInvasives.org contains a plethora of helpful guides to the invasive species affecting Vermont’s forests.
- Ten Recommendations for Managing Ash in the Face of Emerald Ash Borer and Climate Change provides practical management recommendations for ash.
- Forest Carbon Markets for Vermont Landowners is an in-depth resource detailing the process of a carbon offset project and specific recommendations for landowners to pursue.
- Climate Change Response Framework describes resources on forest adaptation and access to the forest adaptation workbook.
- Adaptive Silviculture for Climate Change provides operational examples of adaptive silviculture in action.
- Forest Impacts of Climate Change: Monitoring Indicators includes climate-change indicators for Vermont, New York and greater New England across categories like aquatic systems, forest systems, trees and wildlife.
2.12 References
- American Forests. (2021). Tree equity score. https://www.treeequityscore.org/
- Baldwin, R.F., Fouch, N.T. (2018). Understanding the biodiversity contributions of small, protected areas presents many challenges. Land 7, 123. https://doi.org/10.3390/land7040123
- Berlik, M.M., Kittredge, D.B., Foster, D.R. (2002). The illusion of preservation: a global environmental argument for the local production of natural resources. Journal of Biogeography 29, 1557–1568.
- Bick, S., Berry, A.H., Frederick, P., Steele, A. (2019). Climate adaptation in the northeast’s forest products supply chain: a vulnerability assessment of the primary forest products sector.
- Bradford, J.B., D’Amato, A.W. (2012). Recognizing trade-offs in multi-objective land management. Frontiers in Ecology and the Environment 10, 210–216. https://doi.org/10.1890/110031
- Buermann, W., Bikash, P.R., Jung, M., Burn, D.H., Reichstein, M. (2013). Earlier springs decrease peak summer productivity in North American boreal forests. Environ. Res. Lett. 8, 024027. https://doi.org/10.1088/1748-9326/8/2/024027
- Butler, B.J., Caputo, J., Robillard, A.L., Sass, E.M., Sutherland, C. (2021). One size does not fit all: relationships between size of family forest holdings and owner attitudes and behaviors. Journal of Forestry 119, 28–44. https://doi.org/10.1093/jofore/fvaa045
- Cale, J.A., Teale, S.A., Johnston, M.T., Boyer, G.L., Perri, K.A., Castello, J.D. (2015). New ecological and physiological dimensions of beech bark disease development in aftermath forests. Forest Ecology and Management 336, 99–108. https://doi.org/10.1016/j.foreco.2014.10.019
- Campbell, J., Rustad, L.E., Boyer, E.W., Christopher, S.F., Driscoll, C.T., Fernandez, I., Groffman, P.M., Houle, D., Kiekbusch, J., Magill, A.H., Mitchell, M.J., & Ollinger, S.V. (2009). Consequences of climate change for biogeochemical cycling in forests of northeastern North America. Canadian Journal of Forest Research 39.
- Campbell, J.L., Socci, A.M., & Templer, P.H. (2014). Increased nitrogen leaching following soil freezing is due to decreased root uptake in a northern hardwood forest. Global Change Biology 20, 2663–2673. https://doi.org/10.1111/gcb.12532
- Catanzaro, P., D’Amato, A., & Huff, E.S. (2016). Increasing Forest Resiliency for an Uncertain Future.
- Catanzaro, P., & D’Amato, A.W. (2019). Forest Carbon: An essential natural solution for climate change.
- Clark, P., & D’Amato, T. (2019). Germination, Phenology, and Plant Allocation Patterns of Northeastern Tree Seedlings in Response to Future Precipitation Scenarios.
- Classen, A.T., Sundqvist, M.K., Henning, J.A., Newman, G.S., Moore, J.A.M., Cregger, M.A., Moorhead, L.C., & Patterson, C.M. (2015). Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead? Ecosphere 6, art130. https://doi.org/10.1890/ES15-00217.1
- Climate Change Response Framework. (2020). Vermont Land Trust: Increasing Opportunities for Sustainable Timber Harvest on the Atlas Timberlands [WWW Document]. URL https://forestadaptation.org/adapt/demonstration-projects/vermont-land-trust-increasing-opportunities-sustainable-timber-harvest (accessed 6.9.21).
- Cogbill, C.V., Burk, J., & Motzkin, G. (2002). The forests of presettlement New England, USA: spatial and compositional patterns based on town proprietor surveys. Journal of Biogeography 29, 1279–1304. https://doi.org/10.1046/j.1365-2699.2002.00757.x
- Contosta, A.R., Adolph, A., Burchsted, D., Burakowski, E., Green, M., Guerra, D., Albert, M., Dibb, J., Martin, M., McDowell, W.H., Routhier, M., Wake, C., Whitaker, R., & Wollheim, W. (2017). A longer vernal window: the role of winter coldness and snowpack in driving spring transitions and lags. Global Change Biology 23, 1610–1625. https://doi.org/10.1111/gcb.13517
- Contosta, A.R., Casson, N.J., Garlick, S., Nelson, S.J., Ayres, M.P., Burakowski, E.A., Campbell, J., Creed, I., Eimers, C., Evans, C., Fernandez, I., Fuss, C., Huntington, T., Patel, K., Sanders‐DeMott, R., Son, K., Templer, P., & Thornbrugh, C. (2019). Northern forest winters have lost cold, snowy conditions that are important for ecosystems and human communities. https://doi.org/10.1002/eap.1974
- Couture, J.J., Meehan, T.D., Kruger, E.L., & Lindroth, R.L. (2015). Insect herbivory alters impact of atmospheric change on northern temperate forests. Nature Plants 1, 1–5. https://doi.org/10.1038/nplants.2015.16
- Dale, V.H., Joyce, L.A., McNulty, S., Neilson, R.P., Ayres, M.P., Flannigan, M.D., Hanson, P.J., Irland, L.C., Lugo, A.E., Peterson, C.J., Simberloff, D., Swanson, F.J., Stocks, B.J., & Wotton, B.M. (2001). Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience 51, 723–734. https://doi.org/10.1641/0006-3568(2001)051[0723:CCAFD]2.0.CO;2
- D’Amato, A.W., Mahaffey, A., Pepper, L., Kosiba, A., Patch, N., & van Loon, P. (2020). Ten Recommendations for Managing Ash in the Face of Emerald Ash Borer and Climate Change 3.
- D’Amato, A.W. & Palik, B.J. (2020). Building on the last “new” thing: exploring the compatibility of ecological and adaptation silviculture. Canadian Journal of Forest Research 142. https://doi.org/10.1139/cjfr-2020-0306
- Davidson, C.B., Gottschalk, K.W., & Johnson, J.E. (1999). Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: a review. Forest Science 45:1. https://doi.org/10.1093/forestscience/45.1.74 \
- DeHayes, D.H., Schaberg, P.G., Hawley, G.J., & Strimbeck, G.R. (1999). Acid rain impacts on calcium nutrition and forest health: Alteration of membrane-associated calcium leads to membrane destabilization and foliar injury in red spruce. BioScience 49, 789–800. https://doi.org/10.2307/1313570
- Dobson, A. & Blossey, B. (2015). Earthworm invasion, white-tailed deer, and seedling establishment in deciduous forests of north-eastern North America. J Ecol 103, 153–164. https://doi.org/10.1111/1365-2745.12350
- Domke, G.M., Walters, B.F., Nowak, D.J., Smith, J., Ogle, S.M., Coulston, J.W., & Wirth, T.C., (2020). Greenhouse gas emissions and removals from forest land, woodlands, and urban trees in the United States, 1990-2018. https://doi.org/10.2737/FS-RU-227
- Dugan, A.J., Lichstein, J.W., Steele, A., Metsaranta, J.M., Bick, S., & Hollinger, D.Y. (2021). Opportunities for forest sector emissions reductions: a state-level analysis. Ecological Applications n/a, e02327. https://doi.org/10.1002/eap.2327
- Dukes, J.S., Pontius, J., Orwig, D., Garnas, J.R., Rodgers, V.L., Brazee, N., Cooke, B., Theoharides, K.A., Stange, E.E., Harrington, R., Ehrenfeld, J., Gurevitch, J., Lerdau, M., Stinson, K., Wick, R., & Ayres, M. (2009). Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 39, 231–248. https://doi.org/10.1139/X08-171
- Duveneck, M.J. & Thompson, J.R. (2017). Climate change imposes phenological trade-offs on forest net primary productivity. Journal of Geophysical Research: Biogeosciences 122, 2298–2313. https://doi.org/10.1002/2017JG004025
- Duveneck, M.J., Thompson, J.R., Gustafson, E.J., Liang, Y., & Bruijn, A.M.G. de. (2016). Recovery dynamics and climate change effects to future New England forests. Landscape Ecol 32, 1385–1397. https://doi.org/10.1007/s10980-016-0415-5
- Elkinton, J., Boettner, G., Liebhold, A.M., & Gwiazdowski, R. (2015). Biology, spread, and biological control of winter moth in the eastern United States. 24.
- Emerald Ash Borer (EAB) Infested Area in Vermont. (2021).
- EPA. (2021). Greenhouse Gas Equivalencies Calculator [WWW Document]. U.S. Environmental Protection Agency Energy and Environment. URL https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator (accessed 6.8.21).
- Fahey, R.T., Bialecki, M.B., & Carter, D.R. (2013). Tree growth and resilience to extreme drought across an urban land-use gradient. Arboriculture and Urban Forestry 39, 279–285.
- FEMC. (2019). Dataset: Carbon storage and Net Growth of the Northeastern Forests. Forest Ecosystem Monitoring Cooperative – University of Vermont.
- Fidel, J., McCarthy, K., & Voigt, B. (2018). Tracking parcelization over time: updating the Vermont database to inform planning and policy (Phase III Report).
- Fisichelli, N., Frelich, L.E., & Reich, P.B. (2012). Sapling growth responses to warmer temperatures ‘cooled’ by browse pressure. Global Change Biology 18, 3455–3463. https://doi.org/10.1111/j.1365-2486.2012.02785.x
- Fisichelli, N.A., Abella, S.R., Peters, M., & Krist, F.J. (2014). Climate, trees, pests, and weeds: Change, uncertainty, and biotic stressors in eastern U.S. national park forests. Forest Ecology and Management 327, 31–39. https://doi.org/10.1016/j.foreco.2014.04.033
- Foran, C.M., Baker, K.M., Narcisi, M.J., & Linkov, I. (2015). Susceptibility assessment of urban tree species in Cambridge, MA, from future climatic extremes. Environ Syst Decis 35, 389–400. https://doi.org/10.1007/s10669-015-9563-4
- Franklin, J.F., Spies, T.A., Pelt, R.V., Carey, A.B., Thornburgh, D.A., Berg, D.R., Lindenmayer, D.B., Harmon, M.E., Keeton, W.S., Shaw, D.C., Bible, K., & Chen, J. (2002). Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. Forest Ecology and Management, Forest Ecology in the next millennium: Putting the long view into Practice 155, 399–423. https://doi.org/10.1016/S0378-1127(01)00575-8
- Frey, S.D., Ollinger, S., Nadelhoffer, K., Bowden, R., Brzostek, E., Burton, A., Caldwell, B.A., Crow, S., Goodale, C.L., Grandy, A.S., Finzi, A., Kramer, M.G., Lajtha, K., LeMoine, J., Martin, M., McDowell, W.H., Minocha, R., Sadowsky, J.J., Templer, P.H., & Wickings, K. (2014). Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121, 305–316. https://doi.org/10.1007/s10533-014-0004-0
- Gillespie, N., Unthank, A., Campbell, L., Anderson, P., Gubernick, R., Weinhold, M., Cenderelli, D., Austin, B., McKinley, D., Wells, S., Rowan, J., Orvis, C., Hudy, M., Bowden, A., Singler, A., Fretz, E., Levine, J., & Kirn, R. (2014). Flood Effects on Road–Stream Crossing Infrastructure: Economic and Ecological Benefits of Stream Simulation Designs. Fisheries 39, 62–76. https://doi.org/10.1080/03632415.2013.874527
- Graves, R.A., Haugo, R.D., Holz, A., Nielsen-Pincus, M., Jones, A., Kellogg, B., Macdonald, C., Popper, K., & Schindel, M. (2020). Potential greenhouse gas reductions from natural climate solutions in Oregon, USA. PLOS ONE 15, e0230424. https://doi.org/10.1371/journal.pone.0230424
- Groffman, P.M., Rustad, L.E., Templer, P.H., Campbell, J.L., Christenson, L.M., Lany, N.K., Socci, A.M., Vadeboncoeur, M.A., Schaberg, P.G., Wilson, G.F., Driscoll, C.T., Fahey, T.J., Fisk, M.C., Goodale, C.L., Green, M.B., Hamburg, S.P., Johnson, C.E., Mitchell, M.J., Morse, J.L., Pardo, L.H., & Rodenhouse, N.L. (2012). Long-term integrated studies show complex and surprising effects of climate change in the northern hardwood forest. BioScience 62, 1056–1066. https://doi.org/10.1525/bio.2012.62.12.7
- Guilbert, J., Beckage, B., Winter, J.M., Horton, R.M., Perkins, T., & Bomblies, A. (2014). Impacts of projected climate change over the Lake Champlain Basin in Vermont. Journal of Applied Meteorology and Climatology 53, 1861–1875. https://doi.org/10.1175/JAMC-D-13-0338.1
- Gundersen, P., Thybring, E.E., Nord-Larsen, T., Vesterdal, L., Nadelhoffer, K.J., & Johannsen, V.K. (2021). Old-growth forest carbon sinks overestimated. Nature 591, E21–E23. https://doi.org/10.1038/s41586-021-03266-z
- Halman, J., & Wilmot, S. (2017). Early averages of sugar maple bud development stages.
- Hancock, C. (2020). Forest carbon: a natural climate solution and tool for advancing the pace of conservation. Cold Hollow to Canada. URL https://www.coldhollowtocanada.org/what/news/article/forest-carbon-a-natural-climate-solution-and-tool-for-advancing-the-pace-of-conservation (accessed 6.10.21).
- Handler, S.D., Pike, C., & St. Clair, B. (2018). Assisted Migration | USDA Climate Change Resource Center. URL https://www.fs.usda.gov/ccrc/topics/assisted-migration (accessed 6.9.21).
- Holzmueller, E.J., Jose, S., & Jenkins, M.A. (2010). Ecological consequences of an exotic fungal disease in eastern U.S. hardwood forests. Forest Ecology and Management 259, 1347–1353. https://doi.org/10.1016/j.foreco.2010.01.014
- Hrinkevich, K.H., Progar, R.A., & Shaw, D.C. (2016). Climate risk modelling of balsam woolly adelgid damage severity in subalpine fir stands of western North America. PLOS ONE 11, e0165094. https://doi.org/10.1371/journal.pone.0165094
- Huang, J., Qu, B., Fang, G., Li, X., & Zong, S. (2020). The drivers of the Asian long horned beetle disaster show significant spatial heterogeneity. Ecological Indicators 117, 106680. https://doi.org/10.1016/j.ecolind.2020.106680
- Hufkens, K., Friedl, M., Sonnentag, O., Braswell, B.H., Milliman, T., & Richardson, A.D. (2012). Linking near-surface and satellite remote sensing measurements of deciduous broadleaf forest phenology. Remote Sensing of Environment, Remote Sensing of Urban Environments 117, 307–321. https://doi.org/10.1016/j.rse.2011.10.006
- IPCC. (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland.
- i-Tree, 2021. i-Tree. URL itreetools.org (accessed 6.8.21).
- Iverson, L., Prasad, A., & Matthews, S. (2008). Modeling potential climate change impacts on the trees of the northeastern United States. Mitig Adapt Strateg Glob Change 13, 487–516. https://doi.org/10.1007/s11027-007-9129-y
- Iverson, L.R., Prasad, A.M., Peters, M.P., & Matthews, S.N. (2019). Facilitating adaptive forest management under climate change: a spatially specific synthesis of 125 species for habitat changes and assisted migration over the eastern United States. Forests 10, 989. https://doi.org/10.3390/f10110989
- Janowiak, M.K., D’Amato, A.W., Swanston, C.W., Iverson, L., Thompson, F.R., Dijak, W.D., Matthews, S., Peters, M.P., Prasad, A., Fraser, J.S., Brandt, L.A., Butler-Leopold, P., Handler, S.D., Shannon, P.D., Burbank, D., Campbell, J., Cogbill, C., Duveneck, M.J., Emery, M.R., Fisichelli, N., Foster, J., Hushaw, J., Kenefic, L., Mahaffey, A., Morelli, T.L., Reo, N.J., Schaberg, P.G., Simmons, K.R., Weiskittel, A., Wilmot, S., Hollinger, D., Lane, E., Rustad, L., & Templer, P. (2018). New England and northern New York forest ecosystem vulnerability assessment and synthesis: a report from the New England Climate Change Response Framework project (No. NRS-GTR-173). U.S. Department of Agriculture, Forest Service, Northern Research Station, Newtown Square, PA. https://doi.org/10.2737/NRS-GTR-173
- Jeon, S.B., Olofsson, P., & Woodcock, C.E. (2014). Land use change in New England: a reversal of the forest transition. Journal of Land Use Science 9, 105–130. https://doi.org/10.1080/1747423X.2012.754962
- Jones, M.I., Gould, J.R., Mahon, H.J., & Fierke, M.K. (2020). Phenology of Emerald Ash Borer (Coleoptera: Buprestidae) and Its Introduced Larval Parasitoids in the Northeastern United States. Journal of Economic Entomology 113, 622–632. https://doi.org/10.1093/jee/toz304
- Keenan, T.F., Gray, J., Friedl, M.A., Toomey, M., Bohrer, G., Hollinger, D.Y., Munger, J.W., O’Keefe, J., Schmid, H.P., Wing, I.S., Yang, B., & Richardson, A.D. (2014). Net carbon uptake has increased through warming-induced changes in temperate forest phenology. Nature Clim Change 4, 598–604. https://doi.org/10.1038/nclimate2253
- Keeton, W.S. (2006). Managing for late-successional/old-growth characteristics in northern hardwood-conifer forests. Forest Ecology and Management 235, 129–142. https://doi.org/10.1016/j.foreco.2006.08.005
- Kikuchi, T., Kobayashi K. Sakata, K. Akasaka, T. 2012. Gypsy moth-induced dermatitis: a hospital review and community survey. European Journal of Dermatology 22:3. https://doi.org/10.1684/ejd.2012.1722
- Kittredge, D.B. (2005). The cooperation of private forest owners on scales larger than one individual property: international examples and potential application in the United States. Forest Policy and Economics 7, 671–688. https://doi.org/10.1016/j.forpol.2003.12.004
- Kosiba, A.M. (2021a). Vermont Forest Carbon Inventory.
- Kosiba, A.M. (2021b). Forest Carbon Offsets for Vermont Landowners.
- Kosiba, A.M., Schaberg, P.G., Rayback, S.A., & Hawley, G.J. (2018). The surprising recovery of red spruce growth shows links to decreased acid deposition and elevated temperature. Science of The Total Environment 637–638, 1480–1491. https://doi.org/10.1016/j.scitotenv.2018.05.010
- Kuehn, D., Chase, L., & Sharkey, T. (2017). Adapting to Climate Change: Perceptions of Maple Producers in New York and Vermont. Journal of Agriculture, Food Systems, and Community Development 7, 43–65. https://doi.org/10.5304/jafscd.2017.073.020
- Kuloglu, T.Z., Lieffers, V.J., & Anderson, A.E. (2019). Impact of Shortened Winter Road Access on Costs of Forest Operations. Forests 10, 447. https://doi.org/10.3390/f10050447
- Liang, L., & Fei, S. (2014). Divergence of the potential invasion range of emerald ash borer and its host distribution in North America under climate change. Climatic Change 122, 735–746. https://doi.org/10.1007/s10584-013-1024-9
- Livesley, S.J., McPherson, E.G., & Calfapietra, C. (2016). The Urban Forest and Ecosystem Services: Impacts on Urban Water, Heat, and Pollution Cycles at the Tree, Street, and City Scale. Journal of Environmental Quality 45, 119–124. https://doi.org/10.2134/jeq2015.11.0567
- Lovett, G.M., Weiss, M., Liebhold, A.M., Holmes, T.P., Leung, B., Lambert, K.F., Orwig, D.A., Campbell, F.T., Rosenthal, J., McCullough, D.G., Wildova, R., Ayres, M.P., Canham, C.D., Foster, D.R., LaDeau, S.L., & Weldy, T. (2016). Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecological Applications 26, 1437–1455. https://doi.org/10.1890/15-1176
- Luyssaert, S., Schulze, E.-D., Börner, A., Knohl, A., Hessenmöller, D., Law, B.E., Ciais, P., & Grace, J. (2008). Old-growth forests as global carbon sinks. Nature 455, 213–215. https://doi.org/10.1038/nature07276
- McAvoy, T.J., Régnière, J., St-Amant, R., Schneeberger, N.F., & Salom, S.M. (2017). Mortality and Recovery of Hemlock Woolly Adelgid (Adelges tsugae) in Response to Winter Temperatures and Predictions for the Future. Forests 8, 497. https://doi.org/10.3390/f8120497
- McCullough, D.G. (2020). Challenges, tactics, and integrated management of emerald ash borer in North America. Forestry (Land) 93, 197–211. https://doi.org/10.1093/forestry/cpz049
- McHale, M.R., Burke, I.C., Lefsky, M.A., Peper, P.J., & McPherson, E.G. (2009). Urban forest biomass estimates: is it important to use allometric relationships developed specifically for urban trees? Urban Ecosyst 12, 95–113. https://doi.org/10.1007/s11252-009-0081-3
- McMahon, S.M., Parker, G.G., & Miller, D.R. (2010). Evidence for a recent increase in forest growth. PNAS 107, 3611–3615. https://doi.org/10.1073/pnas.0912376107
- McNulty, S.G., & Boggs, J.L. (2010). A conceptual framework: Redefining forest soil’s critical acid loads under a changing climate. Environmental Pollution, Advances of air pollution science: from forest decline to multiple-stress effects on forest ecosystem services 158, 2053–2058. https://doi.org/10.1016/j.envpol.2009.11.028
- McPherson, E.G., Doorn, N.S. van, & Peper, P.J. (2016). Urban tree database and allometric equations. Gen. Tech. Rep. PSW-GTR-253. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 86 p. 253. https://doi.org/10.2737/PSW-GTR-253
- McPherson, E.G., & Simpson, J.R. (1999). Carbon dioxide reduction through urban forestry: guidelines for professional and volunteer tree planters. Gen. Tech. Rep. PSW-GTR-171. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 237 p. 171. https://doi.org/10.2737/PSW-GTR-171
- Messier, C., Bauhus, J., Doyon, F., Maure, F., Sousa-Silva, R., Nolet, P., Mina, M., Aquilué, N., Fortin, M.-J., & Puettmann, K. (2019). The functional complex network approach to foster forest resilience to global changes. Forest Ecosystems 6, 21. https://doi.org/10.1186/s40663-019-0166-2
- Milanović, M., Knapp, S., Pyšek, P., & Kühn, I. (2020). Linking traits of invasive plants with ecosystem services and disservices. Ecosystem Services 42, 101072. https://doi.org/10.1016/j.ecoser.2020.101072
- Millar, C.I., Stephenson, N.L., & Stephens, S.L. (2007). Climate change and forests of the future: managing in the face of uncertainty. Ecological Applications 17, 2145–2151. https://doi.org/10.1890/06-1715.1
- Millward, A.A., Torchia, M., Laursen, A.E., & Rothman, L.D. (2014). Vegetation placement for summer-built surface temperature moderation in an urban microclimate. Environmental Management 53, 1043–1057. https://doi.org/10.1007/s00267-014-0260-8
- Mohan, J.E.M.E., Cox, R.M.C.M., & Iverson, L.R.I.R. (2009). Composition and carbon dynamics of forests in northeastern North America in a future, warmer world. From: NE Forests 2100: A Synthesis of Climate Change Impacts on Forests of the Northeastern US and Eastern Canada. Canadian Journal of Forest Research. https://doi.org/10.1139/X08-185
- Morin, R., 2018. Forestland area by age class.
- Nagel, L.M., Palik, B.J., Battaglia, M.A., D’Amato, A.W., Guldin, J.M., Swanston, C.W., Janowiak, M.K., Powers, M.P., Joyce, L.A., Millar, C.I., Peterson, D.L., Ganio, L.M., Kirschbaum, C., & Roske, M.R. (2017). Adaptive silviculture for climate change: a national experiment in manager-scientist partnerships to apply an adaptation framework. Journal of Forestry; Bethesda 115, 167–178. http://dx.doi.org/10.5849/jof.16-039
- Neumann, J.E., Price, J., Chinowsky, P., Wright, L., Ludwig, L., Streeter, R., Jones, R., Smith, J.B., Perkins, W., Jantarasami, L., & Martinich, J. (2015). Climate change risks to US infrastructure: impacts on roads, bridges, coastal development, and urban drainage. Climatic Change 131, 97–109. https://doi.org/10.1007/s10584-013-1037-4
- Nevins, M.T., D’Amato, A.W., & Foster, J.R. (2021). Future forest composition under a changing climate and adaptive forest management in southeastern Vermont, USA. Forest Ecology and Management 479, 118527. https://doi.org/10.1016/j.foreco.2020.118527
- NIACS. (2021). Adaptation Workbook. USDA Climate Hub’s Northern Institute of Applied Climate Science. URL adaptationworkbook.org (accessed 6.9.21).
- Norby, R.J., Warren, J.M., Iversen, C.M., Medlyn, B.E., & McMurtrie, R.E. (2010). CO2 enhancement of forest productivity constrained by limited nitrogen availability. PNAS 107, 19368–19373. https://doi.org/10.1073/pnas.1006463107
- Nowak, D.J., Coville, R., Endreny, T., Abdi, R., & Van Stan II, J.T. (2020). Valuing Urban Tree Impacts on Precipitation Partitioning, in: Van Stan, I., John T., Gutmann, E., Friesen, J. (Eds.), Precipitation Partitioning by Vegetation: A Global Synthesis. Springer International Publishing, Cham, pp. 253–268. https://doi.org/10.1007/978-3-030-29702-2_15
- Nowak, D.J., & Greenfield, E.J. (2008). Urban and community forests of New England: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, Vermont. Gen. Tech. Rep. NRS-38. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station. 62 p. 38. https://doi.org/10.2737/NRS-GTR-38
- Nowak, D.J., Greenfield, E.J., Hoehn, R.E., & Lapoint, E. (2013). Carbon storage and sequestration by trees in urban and community areas of the United States. Environmental Pollution 178, 229–236. https://doi.org/10.1016/j.envpol.2013.03.019
- Nowak, D.J., Stevens, J.C., Sisinni, S.M., & Luley, C.J. (2002). Effects of urban tree management and species selection on atmospheric carbon dioxide.
- Ollinger, S.V., Richardson, A.D., Martin, M.E., Hollinger, D.Y., Frolking, S.E., Reich, P.B., Plourde, L.C., Katul, G.G., Munger, J.W., Oren, R., Smith, M.-L., U, K.T.P., Bolstad, P.V., Cook, B.D., Day, M.C., Martin, T.A., Monson, R.K., & Schmid, H.P. (2008). Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks. PNAS 105, 19336–19341. https://doi.org/10.1073/pnas.0810021105
- Oswald, E.M., Pontius, J., Rayback, S.A., Schaberg, P.G., Wilmot, S.H., & Dupigny-Giroux, L.-A. (2018). The complex relationship between climate and sugar maple health: Climate change implications in Vermont for a key northern hardwood species. Forest Ecology and Management 422, 303–312. https://doi.org/10.1016/j.foreco.2018.04.014
- Palik, B., D’Amato, A.W., Franklin, J.F., & Johnson, K.N. (2020). Ecological Silviculture: Foundations and Applications. Waveland Press, Incorporated.
- Pardo, L.H., Fenn, M.E., Goodale, C.L., Geiser, L.H., Driscoll, C.T., Allen, E.B., Baron, J.S., Bobbink, R., Bowman, W.D., Clark, C.M., Emmett, B., Gilliam, F.S., Greaver, T.L., Hall, S.J., Lilleskov, E.A., Liu, L., Lynch, J.A., Nadelhoffer, K.J., Perakis, S.S., Robin-Abbott, M.J., Stoddard, J.L., Weathers, K.C., & Dennis, R.L. (2011). Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecological Applications 21, 3049–3082. https://doi.org/10.1890/10-2341.1
- Peters, M.P., Prasad, A.M., Matthews, S.N., & Iverson, L.R. (2020). Climate change tree atlas, Version 4.
- Preisser, E.L., Elkinton, J.S., & Abell, K. (2008). Evolution of increased cold tolerance during range expansion of the elongate hemlock scale Fiorinia externa Ferris (Hemiptera: Diaspididae). Ecological Entomology 33, 709–715. https://doi.org/10.1111/j.1365-2311.2008.01021.x
- Quiring, D., Ostaff, D., Hartling, L., Lavigne, D., Moore, K., & DeMerchant, I. (2008). Temperature and plant hardiness zone influence distribution of balsam woolly adelgid damage in Atlantic Canada. The Forestry Chronicle 84, 558–562. https://doi.org/10.5558/tfc84558-4
- Rapp, J.M., Lutz, D.A., Huish, R.D., Dufour, B., Ahmed, S., Morelli, T.L., & Stinson, K.A. (2019). Finding the sweet spot: Shifting optimal climate for maple syrup production in North America. Forest Ecology and Management 448, 187–197. https://doi.org/10.1016/j.foreco.2019.05.045
- Rayback, S.A., Belmecheri, S., Gagen, M.H., Lini, A., Gregory, R., & Jenkins, C. (2020). North American temperate conifer (Tsuga canadensis) reveals a complex physiological response to climatic and anthropogenic stressors. New Phytologist 228, 1781–1795. https://doi.org/10.1111/nph.16811†
- Robinson, R. (June 10, 2021). Vermont is facing its biggest gypsy moth outbreak in decades. VT Digger. https://vtdigger.org/2021/06/10/vermont-is-facing-its-biggest-gypsy-moth-outbreak-in-decades/
- Rittenhouse, C.D., & Rissman, A.R. (2015). Changes in winter conditions impact forest management in north temperate forests. Journal of Environmental Management 149, 157–167. https://doi.org/10.1016/j.jenvman.2014.10.010
- Royo, A.A., & Carson, W.P. (2006). On the formation of dense understory layers in forests worldwide: consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 36, 1345–1362. https://doi.org/10.1139/x06-025
- Rustad, L., Campbell, J., Dukes, J.S., Huntington, T., Fallon Lambert, K., Mohan, J., & Rodenhouse, N. (2012). Changing climate, changing forests: The impacts of climate change on forests of the northeastern United States and eastern Canada (No. NRS-GTR-99). U.S. Department of Agriculture, Forest Service, Northern Research Station, Newtown Square, PA. https://doi.org/10.2737/NRS-GTR-99
- Schultz, B., Hanson, T., Halman, J., Wilmot, S., Spinney, E., & Greaves, T. (2017). Vermont Forest Carbon Assessment, in: Forest Insect and Disease Conditions in Vermont, 2016. Agency of Natural Resources, Department of Forests, Parks & Recreation, Montpelier, VT.
- Seidl, R., Rammer, W., & Spies, T.A. (2014). Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecological Applications 24, 2063–2077. https://doi.org/10.1890/14-0255.1
- Seymour, R.S. (2005). Integrating natural disturbance parameters into conventional silviculture systems: experience from the Acadian Forest of northeastern North American. In: Balancing Ecosystem Values: Innovative Experiments for Sustainable Forestry. (Charles E. Peterson and Douglas A. Maguire, Eds.) U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station.
- Slippers, B., Groot, P. de, & Wingfield, M.J. (2011). The Sirex Woodwasp and its Fungal Symbiont: Research and Management of a Worldwide Invasive Pest. Springer Science & Business Media.
- Solecki, W.D., & Marcotullio, P.J. (2013). Climate change and urban forest vulnerability, in: Urbanization, Biodiversity, and Ecosystem Services: Challenges and Opportunities. Springer.
- Sperlich, D., Nadal-Sala, D., Gracia, C., Kreuzwieser, J., Hanewinkel, M., & Yousefpour, R. (2020). Gains or Losses in Forest Productivity under Climate Change? The Uncertainty of CO2 Fertilization and Climate Effects. Climate 8, 141. https://doi.org/10.3390/cli8120141
- Steidinger, B.S., Crowther, T.W., Liang, J., Van Nuland, M.E., Werner, G.D.A., Reich, P.B., Nabuurs, G.J., de-Miguel, S., Zhou, M., Picard, N., Herault, B., Zhao, X., Zhang, C., Routh, D., & Peay, K.G. (2019). Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569, 404–408. https://doi.org/10.1038/s41586-019-1128-0
- Stephanson, C.A., & Coe, N.R. (2017). Impacts of Beech Bark Disease and Climate Change. American Beech 17.
- Tubby, K.V., & Webber, J.F. (2010). Pests and diseases threatening urban trees under a changing climate. Forestry: An International Journal of Forest Research 83, 451–459. https://doi.org/10.1093/forestry/cpq027
- Uelmen, J.A., Lindroth, R.L., Tobin, P.C., Reich, P.B., Schwartzberg, E.G., & Raffa, K.F. (2016). Effects of winter temperatures, spring degree-day accumulation, and insect population source on phenological synchrony between forest tent caterpillar and host trees. Forest Ecology and Management 362, 241–250. https://doi.org/10.1016/j.foreco.2015.11.045
- Urban, J.M. (2020). Perspective: shedding light on spotted lanternfly impacts in the USA. Pest Management Science 76, 10–17. https://doi.org/10.1002/ps.5619
- U.S. Census Bureau. (2012). 2010 Census of population and housing, population, and housing unit counts (No. CPH-2-47, Vermont). U.S. Government Printing Office, Washington, D.C.
- US EPA. (2015). Greenhouse Gas Equivalencies Calculator [WWW Document]. US EPA. URL https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator (accessed 6.10.21).
- US EPA. (2014). Improving Indoor Air Quality [WWW Document]. US EPA. URL https://www.epa.gov/indoor-air-quality-iaq/improving-indoor-air-quality (accessed 3.16.21).
- U.S. Forest Service. (2017). EcoMap Sections.
- USDA Forest Service. (2021). Urban FIA Program [WWW Document]. Forest Inventory and Analysis National Program. URL https://www.fia.fs.fed.us/program-features/urban/ (accessed 6.8.21).
- USDA Forest Service. (2020). Forests of Vermont, 2019. Resource Update FS-243 2. https://doi.org/10.2737/FS-RU-243
- USDA Forest Service. (2019). Forests of Vermont, 2018. Resource Update FS–212. Newtown Square, PA: U.S. Department of Agriculture Forest Service, Northern Research Station. 2 p. 212. https://doi.org/10.2737/FS-RU-212
- USDA-APHIS. (2012). English: Dorsal view of emerald ash borer adult with elytra and wings spread.
- USGCRP (2018). Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II: [Reidmiller, D.R., C.W. Avery, D.R. Easterling, K.E. Kunkel, K.L.M. Lewis, T.K. Maycock, and B.C. Stewart (eds.)]. U.S. Global Change Research Program, Washington, DC, USA, 1515 pp. doi: 10.7930/NCA4.2018.
- Vermont Agency of Agriculture, Food & Markets. (2020). 2019 Legislative Summary. Vermont Agency of Agriculture, Food & Markets.
- Vermont Department of Forests, Parks & Recreation. (2018). Forest Tent Caterpillar Update (No. Vermont Forest Health leaflet 2018-13). Vermont Department of Forests, Parks & Recreation.
- Vermont Department of Forests, Parks & Recreation. (June 8, 2021). Gypsy moths are making a comeback in Vermont: caterpillar infestations target tree foliage. https://fpr.vermont.gov/news/gypsy-moths-making-a-comeback-in-vermont
- Vermont Forest Health. (2011). Anthracnose Disease. Vermont Department of Forests, Parks & Recreation.
- Vermont Forest Resources Plan. (2010). Division of Forests, Vermont Department of Forests, Parks and Recreation, Agency of Natural Resources.
- Vermont Sustainable Jobs Fund. (2017). Economic Impact | Vermont Forestry & Wood Products. URL https://www.vsjf.org/impact/forest-products/ (accessed 6.8.21).
- Violle, C., Navas, M.-L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., Garnier, E. (2007). Let the concept of trait be functional! Oikos 116, 882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x
- VT FPR. (n.d.). Stumpage Price Reports | Department of Forests, Parks and Recreation [WWW Document]. URL https://fpr.vermont.gov/stumpage-price-reports (accessed 6.8.21).
- VT UCF. (2021a). Ash Tree Inventories [WWW Document]. Vermont Urban and Community Forestry Inventories and Management Plans. URL https://vtcommunityforestry.org/ash-inventory (accessed 6.8.21).
- VT UCF. (2021b). Tree Canopy Assessments [WWW Document]. Vermont Urban and Community Forestry Inventories and Management Plans. URL https://vtcommunityforestry.org/resources/inventories-management-plans/tree-canopy-assessments (accessed 6.8.21).
- Wilson, G., Green, M., Brown, J., Campbell, J., Groffman, P., Durán, J., & Morse, J. (2020). Snowpack affects soil microclimate throughout the year. Climatic Change 163, 705–722. https://doi.org/10.1007/s10584-020-02943-8
- Zhao, Z., Peng, C., Yang, Q., Meng, F.-R., Song, X., Chen, S., Epule, T.E., Li, P., & Zhu, Q. (2017). Model prediction of biome-specific global soil respiration from 1960 to 2012. Earth’s Future 5, 715–729. https://doi.org/10.1002/2016EF000480
- Zheng, D., Ducey, M.J., & Heath, L.S. (2013). Assessing net carbon sequestration on urban and community forests of northern New England, USA. Urban Forestry & Urban Greening 12, 61–68. https://doi.org/10.1016/j.ufug.2012.10.003

